The electrochemical series is a fundamental concept in chemistry that helps us understand the behavior of different elements and compounds in electrical current and chemical reactions. It is a ranking system that organizes elements based on their tendency to gain or lose electrons in a chemical reaction. This series plays a crucial role in electrochemistry, a branch of chemistry that deals with the relationship between electricity and chemical reactions.
In this article, we will explore 19 mind-blowing facts about the electrochemical series. From the discovery of this concept to its practical applications in various industries, we will delve into the fascinating world of electrochemistry. So, fasten your seatbelts and get ready to be amazed by the incredible properties and characteristics of the elements that make up the electrochemical series.
Key Takeaways:
- The Electrochemical Series ranks elements based on their reactivity and helps us understand how they behave in chemical reactions, like a leaderboard for elements in the chemistry world.
- By using the Electrochemical Series, scientists can predict which elements will play nice together in reactions, design better batteries, and even prevent metal from rusting, like a superhero tool for chemistry problems.
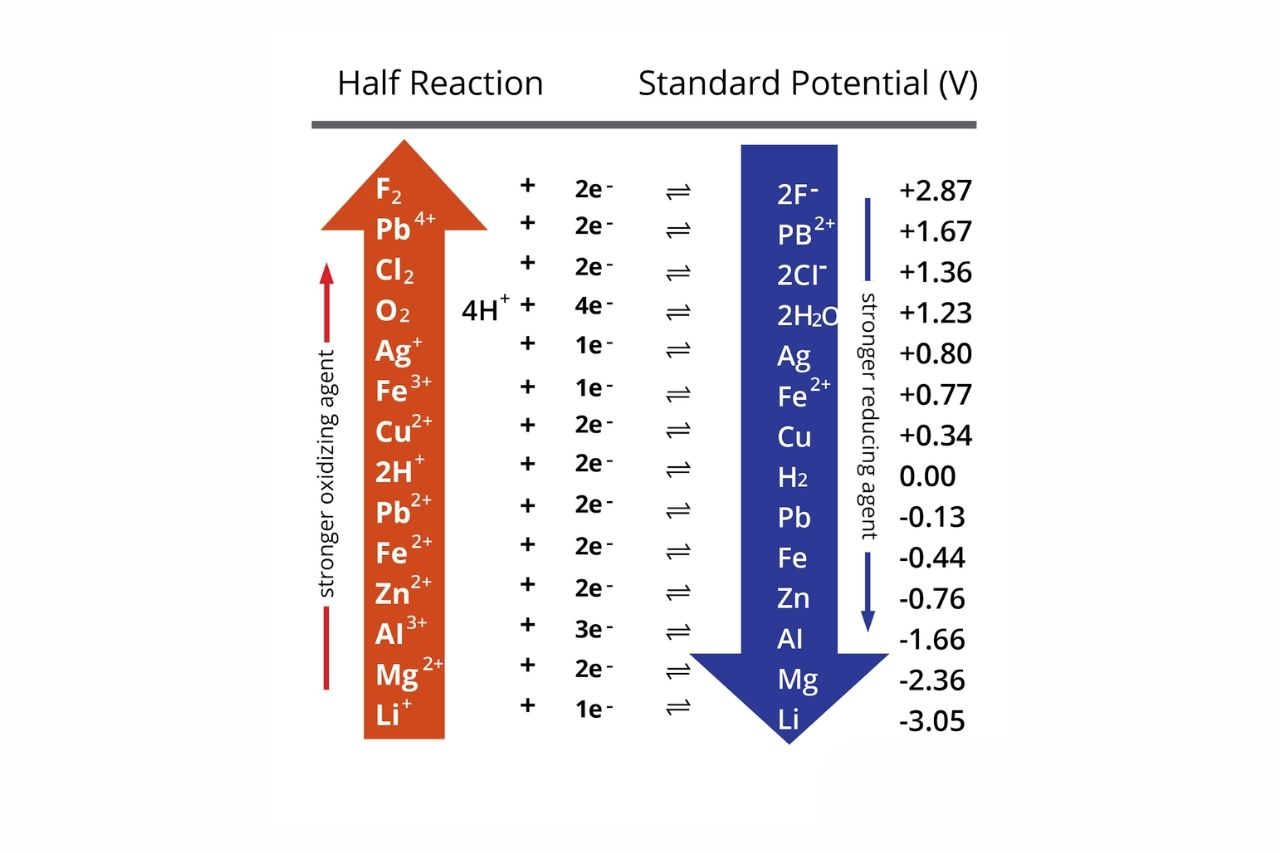
The Electrochemical Series ranks elements based on their electrode potentials.
The Electrochemical Series is a list of elements arranged in order of increasing or decreasing electrode potentials. It allows us to predict the direction of electron flow during redox reactions, as well as the relative reactivity of different elements.
The Electrochemical Series is a tool used in electrochemistry.
Electrochemistry is the study of the interconversion of electrical and chemical energy. The Electrochemical Series helps us analyze and understand redox reactions in batteries, fuel cells, corrosion processes, and other electrochemical systems.
The Electrochemical Series provides a basis for predicting the feasibility of redox reactions.
By comparing the positions of two elements in the Electrochemical Series, we can determine whether a redox reaction between them is spontaneous or non-spontaneous. Elements higher in the series have a greater tendency to undergo reduction, while elements lower in the series have a higher tendency to undergo oxidation.
The Electrochemical Series demonstrates the concept of standard electrode potential.
Standard electrode potential (E°) is the measure of an electrode’s potential with respect to a standard hydrogen electrode. It allows us to determine the relative strengths of oxidizing and reducing agents.
Metals at the top of the Electrochemical Series are strong reducing agents.
Metals like lithium, sodium, and potassium are located at the top of the Electrochemical Series. They have a strong tendency to donate electrons and undergo oxidation, making them excellent reducing agents.
Non-metals at the bottom of the Electrochemical Series are strong oxidizing agents.
Non-metals, such as fluorine and oxygen, are found at the bottom of the Electrochemical Series. They have a high affinity for electrons and tend to undergo reduction, making them powerful oxidizing agents.
The Electrochemical Series is used to calculate cell potentials.
Cell potentials provide important information about the energy available from an electrochemical reaction. By subtracting the standard reduction potential of the anode from the standard reduction potential of the cathode, we can determine the cell potential.
The Electrochemical Series helps predict the reactivity of metals.
Based on their positions in the Electrochemical Series, we can predict the relative reactivity of metals. Metals higher in the series, such as gold and platinum, are less reactive, while metals lower in the series, such as sodium and potassium, are highly reactive.
Transition metals have variable electrode potentials.
Transition metals exhibit a range of electrode potentials due to their ability to form multiple oxidation states. This variability is highlighted in their positions within the Electrochemical Series.
The Electrochemical Series plays a crucial role in designing and optimizing batteries.
Understanding the reactivity of different elements and their electrode potentials helps in the development of efficient and long-lasting batteries. The Electrochemical Series allows scientists to select appropriate electrode materials for specific battery applications.
The Electrochemical Series helps prevent corrosion.
By knowing which metals are more prone to oxidation and corrosion, the Electrochemical Series aids in the selection of appropriate protective measures. It is used to prevent the corrosion of metals through techniques like sacrificial anodes.
The Electrochemical Series can be altered by changing the environment.
Factors such as temperature, concentration, and pressure can influence the electrode potentials and alter the order of elements in the Electrochemical Series. This highlights the dynamic nature of electrochemical reactions.
The Electrochemical Series can be represented by various diagrams.
Common representations of the Electrochemical Series include the galvanic series, the Pourbaix diagram, and the Frost diagram. These diagrams provide a visual representation of the relative positions of different elements.
The Electrochemical Series helps explain phenomena like the rusting of iron.
Iron, when exposed to oxygen and moisture, undergoes oxidation to form iron(III) oxide (rust). The Electrochemical Series explains why iron acts as an anode and exhibits corrosion in this scenario.
The Electrochemical Series is applicable in various industries.
Industries such as metallurgy, energy storage, electroplating, and water treatment rely on the principles of the Electrochemical Series to optimize processes and enhance efficiency.
The Electrochemical Series is a dynamic concept.
The Electrochemical Series is not static and can vary slightly depending on specific conditions. It is essential to consider factors like pH, temperature, and concentration when applying the Electrochemical Series to real-world situations.
The Electrochemical Series finds applications in educational settings.
Understanding the Electrochemical Series is vital for students studying chemistry and electrochemistry. It forms the basis for comprehending concepts such as redox reactions and cell potentials.
The Electrochemical Series continues to be researched and expanded.
Scientists are continuously exploring the properties of elements and their behavior in electrochemical systems. This ongoing research contributes to the expansion and refinement of the Electrochemical Series.
In summary, the Electrochemical Series is a crucial tool in the field of electrochemistry.
It allows us to determine the reactivity of elements, predict the direction of electron flow, calculate cell potentials, and design efficient electrochemical systems. The Electrochemical Series holds immense importance in various industries and continues to be a subject of exploration for scientists worldwide.
Conclusion
The electrochemical series is a fascinating aspect of chemistry that provides valuable insights into the reactivity and behavior of elements and their compounds. This series serves as a reference guide, ranking elements based on their tendency to gain or lose electrons. By understanding this series, scientists and researchers can predict the outcome of various chemical reactions, including those involving electrolysis, batteries, and corrosion. The electrochemical series not only helps in the practical applications of chemistry but also deepens our understanding of the fundamental principles that govern chemical reactions.
FAQs
1. What is the electrochemical series?
The electrochemical series is a table that ranks elements based on their tendency to gain or lose electrons in chemical reactions.
2. How is the electrochemical series useful?
The electrochemical series is useful in predicting the outcome of chemical reactions, such as electrolysis, battery operation, and corrosion.
3. What does the position of an element in the electrochemical series indicate?
The position of an element in the electrochemical series indicates its relative reactivity and its ability to undergo oxidation or reduction reactions.
4. Can the electrochemical series be used to predict the voltage of a cell?
Yes, the electrochemical series can be used to predict the voltage of a galvanic cell. The greater the difference in the position of the two metals in the series, the higher the voltage of the cell.
5. Are there any exceptions to the trends observed in the electrochemical series?
Yes, there can be exceptions to the trends observed in the electrochemical series, especially in the presence of complex ions or unusual conditions.
6. Can the electrochemical series be applied to non-metal elements?
No, the electrochemical series is primarily applicable to metal elements as it is based on their ability to gain or lose electrons.
Unraveling mysteries of electrochemistry doesn't stop with Electrochemical Series. Dive deeper into captivating world of chemical reactions and energy conversion. Explore fascinating realm of redox reactions, where electrons dance between molecules. Discover secrets behind galvanic cells, powerhouses that convert chemical energy into electrical energy. Lastly, prepare to be amazed by electrolysis, process that splits compounds using electricity, with applications ranging from industrial processes to everyday life.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
