Molar volume is a fundamental concept in chemistry that plays a crucial role in understanding the properties of gases. By delving into the intricacies of molar volume, we uncover a world of fascinating facts that shed light on the behavior of gases and their significance in various scientific applications. From its historical significance to its practical implications in everyday life, molar volume is a captivating subject that warrants exploration.
In this article, we will unravel nine extraordinary facts about molar volume, providing insights into its relevance, applications, and the intriguing phenomena associated with it. Whether you're a chemistry enthusiast seeking to deepen your knowledge or simply curious about the wonders of the natural world, these facts are sure to captivate and inspire a newfound appreciation for the intricacies of molar volume. Join us on this enlightening journey as we unveil the remarkable facets of molar volume, delving into its profound impact on the world of chemistry and beyond.
Key Takeaways:
- Molar volume is the space one mole of a substance occupies. It helps chemists understand gases and solids, and is crucial for stoichiometry and materials science.
- Understanding molar volume is key to predicting and explaining the behavior of gases and solids. It influences gas laws and is essential for accurate chemical predictions.
Molar Volume Defined
Molar volume is the space occupied by one mole of any substance at a specific temperature and pressure. It is a crucial concept in chemistry, providing valuable insights into the properties of gases and solids.
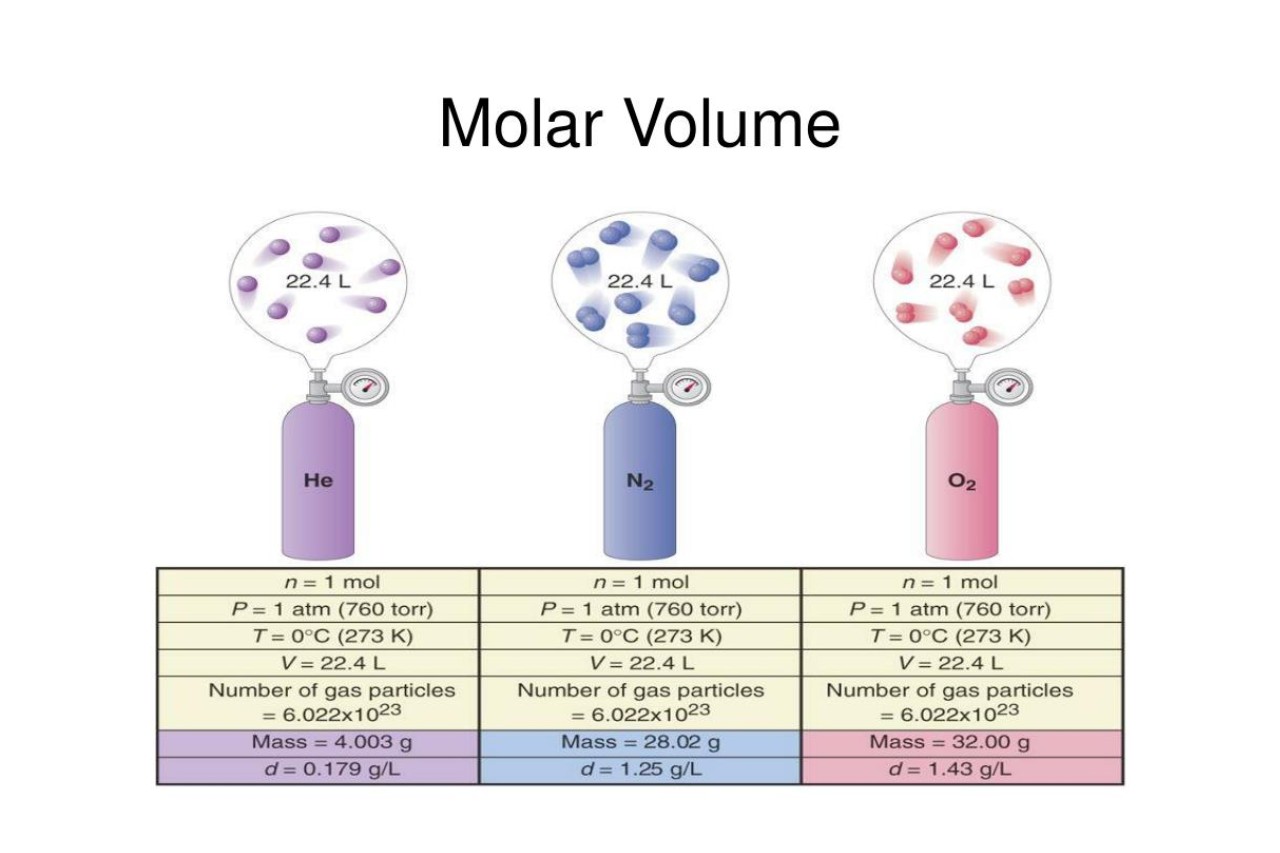
Molar Volume of Ideal Gases
Under standard conditions (0 degrees Celsius and 1 atmosphere pressure), one mole of any ideal gas occupies a volume of 22.4 liters. This relationship is known as the molar volume of ideal gases.
Relationship with Avogadro's Law
According to Avogadro's Law, equal volumes of gases at the same temperature and pressure contain an equal number of molecules. This law directly correlates to the concept of molar volume, highlighting the proportional relationship between volume and the amount of substance.
Application in Stoichiometry
Molar volume plays a pivotal role in stoichiometric calculations, allowing chemists to determine the volume of gases involved in chemical reactions. This is instrumental in understanding and predicting the behavior of gases in various chemical processes.
Deviations from Ideal Behavior
Real gases often deviate from ideal behavior due to intermolecular forces and molecular volume. Understanding these deviations is crucial in accurately predicting the behavior of gases under different conditions.
Molar Volume of Solids
In the context of solids, molar volume refers to the volume occupied by one mole of a solid substance. This parameter provides valuable insights into the arrangement and packing of atoms or molecules in a solid structure.
Importance in Materials Science
Molar volume is a critical parameter in materials science, influencing various properties such as density, compressibility, and thermal expansion. It serves as a fundamental aspect in the characterization and analysis of diverse materials.
Experimental Determination
Experimental techniques, including gas displacement methods and pycnometry, are employed to determine the molar volume of gases and solids. These methods contribute to the accurate measurement and understanding of molar volume in different substances.
Influence on Gas Laws
Molar volume significantly influences the behavior described by gas laws, including Boyle's Law, Charles's Law, and the Ideal Gas Law. Understanding molar volume is essential for comprehending and applying these fundamental principles in chemistry.
Molar volume is truly a remarkable concept in the realm of chemistry, offering profound insights into the behavior of gases and solids. From its fundamental role in stoichiometry to its impact on materials science, the significance of molar volume spans across various domains of chemical research and application. Understanding the concept of molar volume empowers chemists to make accurate predictions and comprehend the underlying principles governing the behavior of substances at the molecular level. Whether analyzing ideal gases or delving into the complexities of real gas behavior, molar volume stands as a cornerstone in the foundation of chemical understanding and experimentation.
Conclusion
In conclusion, molar volume is a fundamental concept in chemistry that plays a crucial role in various scientific calculations and experiments. By understanding the significance of molar volume and its relationship to different elements and compounds, scientists can gain valuable insights into the behavior of substances under specific conditions. The remarkable consistency of molar volume across different gases, as well as its direct correlation to Avogadro's law, highlights the elegance and precision of scientific principles. As researchers continue to explore the intricacies of molar volume, they uncover new possibilities for advancements in chemical analysis, industrial processes, and environmental studies.
FAQs
What is the significance of molar volume in chemistry?Molar volume is essential in chemistry as it provides a direct link between the volume of a substance and the number of moles present. This relationship is crucial for various calculations and experiments, allowing scientists to determine the properties and behaviors of different elements and compounds.
How does molar volume contribute to Avogadro's law?Molar volume is directly related to Avogadro's law, which states that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. This principle underscores the importance of molar volume in understanding the behavior of gases and the fundamental connection between volume and quantity in chemistry.
Molar volume's significance extends beyond chemistry, influencing our understanding of gas laws, <Avogadro's law>, and ideal gas behavior. Gas laws describe pressure, volume, temperature relationships; Avogadro's law connects molar quantities to gas volumes. Ideal gas assumptions simplify calculations but deviate from real-world conditions. Exploring these concepts further reveals molar volume's profound impact on scientific understanding.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
