Esterification is a chemical reaction that plays a fundamental role in various industries, including pharmaceuticals, fragrance production, and food flavoring. It involves the synthesis of esters, which are compounds with a wide range of applications and distinctive properties. While many may think that esterification is a straightforward process, there are several surprising facts about it that are worth exploring.
In this article, we will delve into 18 surprising facts about esterification, shedding light on its significance, mechanism, applications, and even its impact on our everyday lives. From its historic origins to its modern-day applications, prepare to be amazed by the fascinating world of esterification.
Key Takeaways:
- Esterification is a cool chemical process that makes things smell fruity, creates biodiesel, and even helps make your favorite cosmetics and plastics. It’s like a magical scent and substance creator!
- Esterification is a versatile chemical reaction that’s been around for centuries. It’s used to make everything from perfumes to pharmaceuticals, and it can even be reversed under certain conditions. It’s like a secret ingredient in so many everyday products!
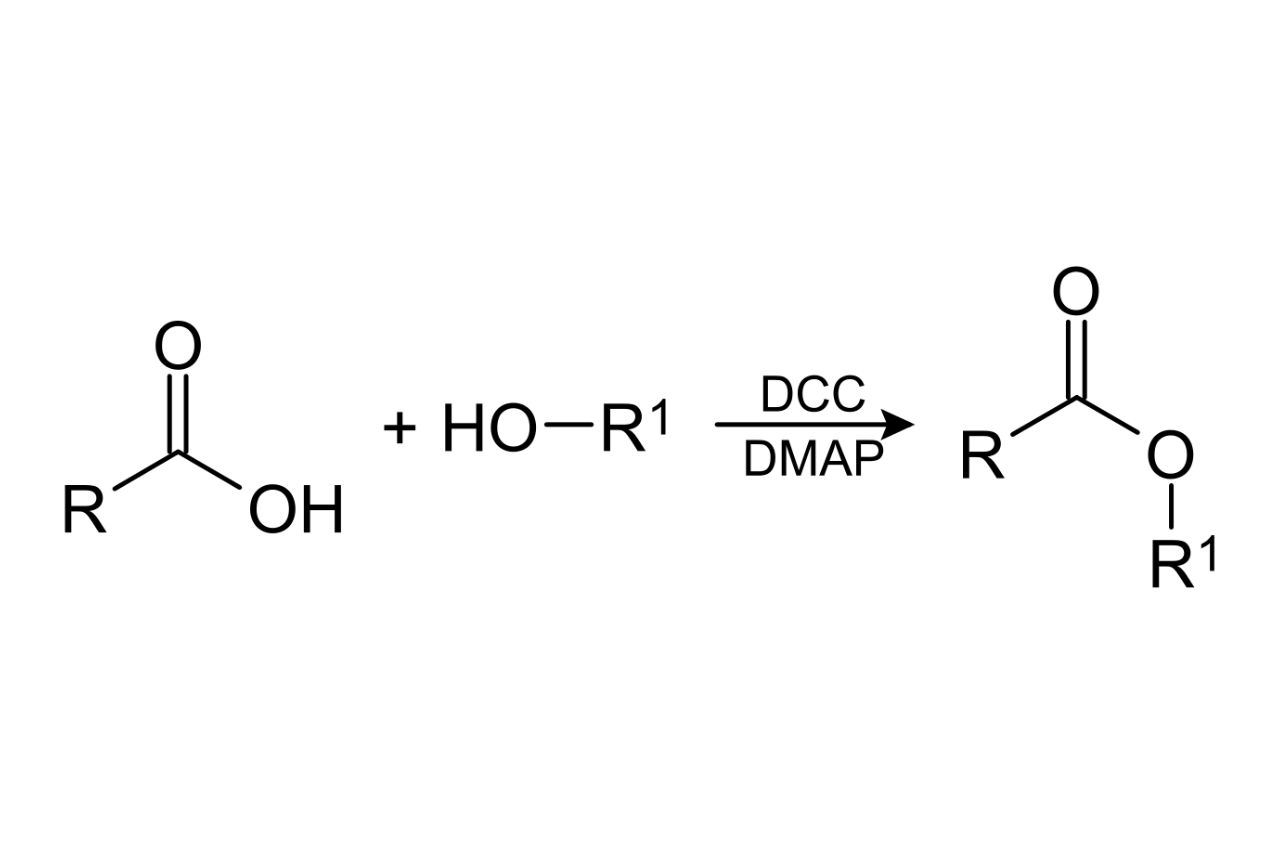
Esterification involves the reaction between an alcohol and an acid.
Esterification is a condensation reaction where an alcohol (containing an -OH group) reacts with an acid (containing a carboxyl group) to form an ester and water as a byproduct. This reaction is catalyzed by an acid or an enzyme.
Esterification is responsible for the fruity smell of many fruits.
The characteristic smell of fruits is often the result of esterification reactions. For example, the sweet aroma of bananas is due to the ester called isoamyl acetate, while the scent of pineapples is attributed to ethyl butyrate.
Esterification is used in the production of biodiesel.
Through esterification, vegetable oils or animal fats can be converted into biodiesel, which is a renewable and environmentally friendly fuel source.
Esterification is a reversible reaction.
Under certain conditions, esterification can be reversed, enabling the production of both esters and alcohols from a single reaction mixture.
Esterification is used in the production of perfumes and fragrances.
The enticing scents of perfumes and fragrances are often created through the synthesis of esters, which contribute to the overall aroma and longevity of the product.
Esterification plays a role in the preservation of food.
By forming esters with fatty acids, esterification helps to extend the shelf life of certain food products and prevent rancidity.
Esterification is involved in the synthesis of polymers.
Esterification reactions are crucial in the production of various polymers, including polyesters, which are widely used in textiles, packaging materials, and even biomedical applications.
Esterification is used in the production of solvents.
Many solvents, such as ethyl acetate and methyl ethyl ketone, are synthesized through esterification reactions. These solvents find applications in paints, coatings, and other industrial processes.
Esterification plays a role in the production of soaps and detergents.
The esterification of fatty acids with alcohols is an essential step in the production of soaps and detergents, giving them their cleansing properties.
Esterification reactions can be influenced by temperature and catalysts.
Changing the reaction temperature or using different catalysts can significantly impact the rate and outcome of esterification reactions.
Esterification is commonly used in the synthesis of pharmaceuticals.
Many pharmaceutical compounds are synthesized through esterification reactions, which allow for the incorporation of specific functional groups and enhance the drug’s stability and bioavailability.
Esterification reactions are widely studied in organic chemistry.
Due to their importance and versatility, esterification reactions are extensively researched and taught in organic chemistry curricula.
Esterification can occur in both acidic and enzymatic environments.
While traditional esterification reactions often involve acidic conditions, enzymatic esterification offers a milder and more sustainable approach.
Esterification reactions can be influenced by the surrounding pressure.
By adjusting the pressure, the equilibrium of an esterification reaction can be shifted to favor the formation of esters or alcohols.
Esterification is utilized in the production of plasticizers.
Plasticizers, which are additives used to improve the flexibility and durability of plastics, are often synthesized through esterification reactions.
Esterification reactions can be accelerated by ultrasonic waves.
Applying ultrasonic waves to an esterification reaction can enhance the efficiency and speed of the reaction, reducing the reaction time required.
Esterification plays a role in the production of cosmetics.
The formulation of cosmetics, including creams, lotions, and lipsticks, utilizes esterification reactions to create products with desired textures and properties.
Esterification reactions have been known for centuries.
The concept of esterification has been recognized and utilized since ancient times, with early records of its application found in ancient Egyptian and Roman civilizations.
Esterification is a versatile and fascinating chemical process with countless applications across various industries. From creating delightful fragrances to synthesizing polymers and biofuels, esterification continues to play a pivotal role in shaping our everyday lives. These 18 surprising facts about esterification showcase its importance and highlight its significant contributions to various fields of study. So, the next time you encounter a pleasant scent or a product with unique properties, remember that it might be the result of the incredible esterification process.
Conclusion
In conclusion, esterification is a fascinating chemical process that has numerous applications in various industries. It involves the reaction between an alcohol and an organic acid to produce an ester and water. Esterification plays a vital role in the production of perfumes, flavors, solvents, and pharmaceuticals.
Throughout this article, we have discussed 18 surprising facts about esterification, highlighting its importance and versatility. From its historical significance to its impact on our daily lives, esterification continues to captivate chemists and researchers alike.
Whether you are a chemistry enthusiast, a student, or simply curious about the world of molecules, understanding the intricacies of esterification can deepen your appreciation for the wonders of chemistry.
FAQs
1. What is esterification?
Esterification is a chemical process in which an alcohol and an organic acid react to form an ester and water.
2. What are some common uses of esterification?
Esterification has various applications, including the production of perfumes, flavors, solvents, and pharmaceuticals.
3. Can you provide an example of esterification?
Sure! An example of esterification is when ethanol (alcohol) reacts with acetic acid to form ethyl acetate, which gives the characteristic smell of vinegar.
4. Are there any safety precautions to take during esterification?
Yes, it is essential to handle organic acids and alcohols with caution as they may be flammable or toxic. Proper ventilation and personal protective equipment should be used when conducting esterification reactions.
5. Can esterification occur without a catalyst?
Yes, esterification can occur without a catalyst; however, the reaction rate is significantly slower. The addition of a catalyst, such as sulfuric acid, can help accelerate the esterification process.
Esterification's fascinating facts barely scratch the surface of chemistry's wonders. Dive deeper into alcohols' versatile nature, uncover carboxylic acids' astounding properties, and explore catalysis' enigmatic influence on chemical reactions. Each topic offers a unique perspective on the intricate world of chemistry, inviting curious minds to expand their knowledge and appreciate the science behind everyday phenomena. So, whether you're a budding chemist or simply curious about the world around you, these captivating subjects promise to enlighten and inspire.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
