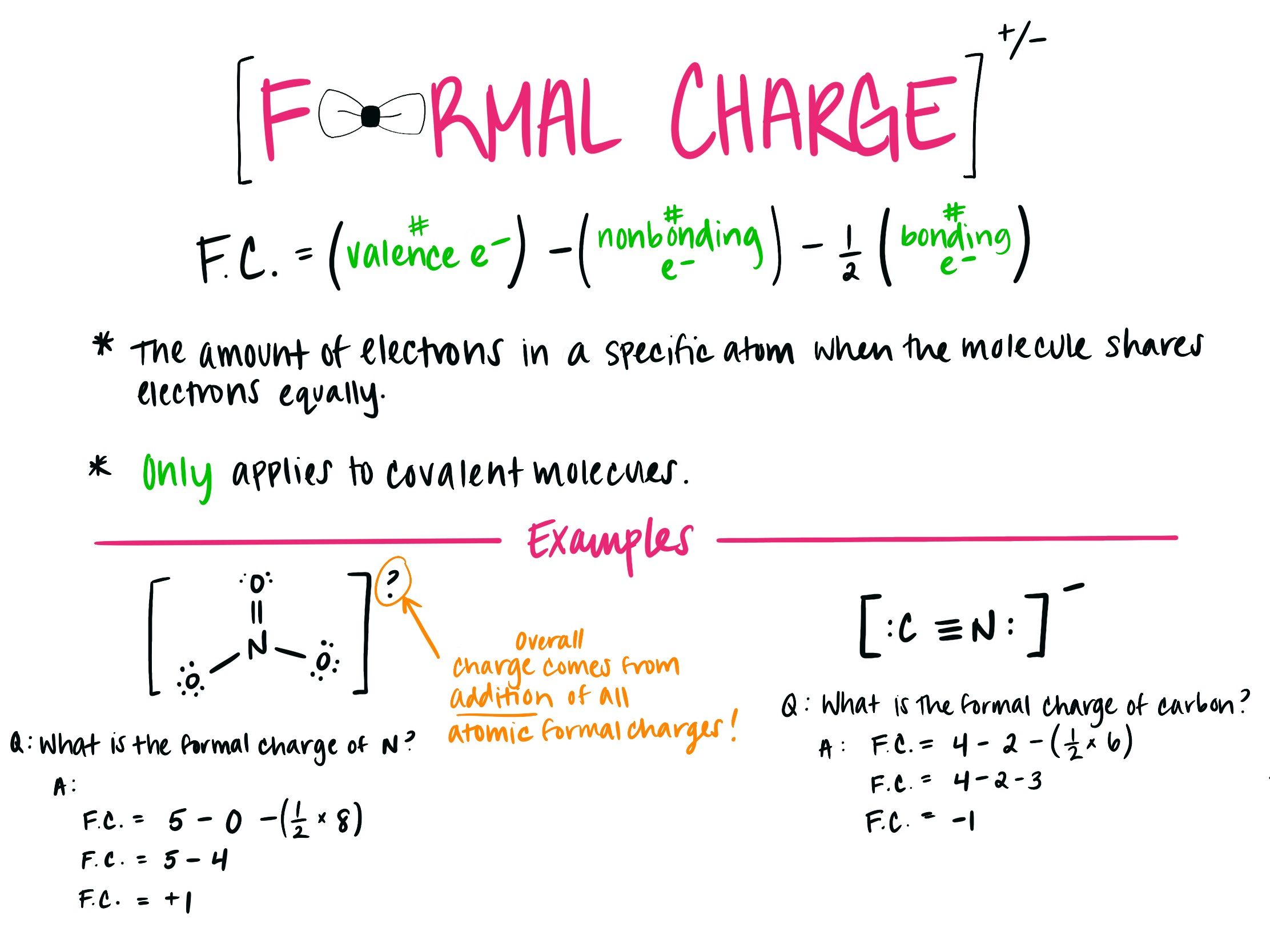
Formal charge is a concept in chemistry that helps us understand the distribution of electrons within a molecule or an ion. It is a handy tool for predicting the stability, reactivity, and overall behavior of chemical species. While it may sound complex, formal charge is actually quite fascinating and can reveal some captivating insights.
In this article, we will delve into the world of formal charge and explore 18 intriguing facts that will deepen your understanding of this fundamental concept. From understanding the basics of formal charge to its significance in Lewis structures and resonance, we will unravel the mysteries behind this concept step by step.
So, whether you are a chemistry enthusiast looking to expand your knowledge or a student preparing for an exam, get ready to explore the captivating world of formal charge and unveil its secrets!
Key Takeaways:
- Formal charge helps us understand how electrons are distributed in molecules and predicts their stability and reactivity. It’s like a map that guides chemists in predicting how molecules will behave in chemical reactions.
- By looking at formal charges, chemists can figure out the shape, stability, and reactivity of molecules. It’s like solving a puzzle to uncover the secrets of how molecules interact and change.
What is Formal Charge?
Formal charge is a measure of the distribution of electrons in a molecule or ion. It helps us understand the relative abundance or deficiency of electrons on each atom and aids in predicting chemical behavior.
How is Formal Charge Calculated?
Formal charge is calculated by assigning valence electrons to the atoms in a molecule and comparing them to the number of electrons each atom actually possesses.
What Does a Formal Charge of Zero Indicate?
A formal charge of zero on an atom signifies that the atom has an electron count that is consistent with its neutral, isolated state.
The Importance of Formal Charge in Resonance Structures
Formal charge is crucial in understanding resonance structures, which are multiple valid representations of a molecule or ion that differ in the arrangement of electrons.
Formal Charges and Stability
Molecules with lower formal charges on individual atoms are generally more stable. This is because a more balanced distribution of electrons reduces electrostatic repulsion and improves stability.
Relationship between Formal Charge and Oxidation State
Formal charge and oxidation state are interconnected but not equivalent. Formal charge focuses on the distribution of electrons within a molecule, while oxidation state relates to the transfer of electrons between atoms.
The Impact of Formal Charge on Chemical Reactivity
Formal charge influences the reactivity of atoms within a molecule. Atoms with higher formal charges are more likely to participate in chemical reactions due to their electron deficiency or abundance.
Formal Charge in Coordination Complexes
Formal charge is particularly relevant in coordination complexes, where transition metals form complexes with ligands. Understanding the formal charges of the ligands helps predict the stability and structure of these complexes.
The Role of Formal Charge in Organic Chemistry
Formal charge is an indispensable tool for understanding the stability and reactivity of organic compounds. It aids in predicting the behavior of functional groups, resonance structures, and reaction mechanisms.
Formal Charge and Molecular Geometry
Formal charge plays a role in determining the shape of molecules. It influences the position of lone pairs and bonding electrons, affecting the overall geometry of the molecule.
Formal Charge and Lewis Structures
Formal charge is used in the construction of Lewis structures, which are visual representations of molecules that illustrate the connectivity of atoms and the distribution of electrons.
Formal Charge in Electrostatic Potential Maps
Using formal charge, scientists can create electrostatic potential maps, which provide visual representations of electron density and help determine the regions of high and low electron density in a molecule.
Formal Charge and Molecular Stability
Formal charge aids in understanding the stability of molecules. It helps identify charges that may lead to instability or unusual reactivity, providing insight into the overall behavior of the compound.
Formal Charge and VSEPR Theory
Formal charge is closely connected to the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the three-dimensional shape of molecules based on repulsion between electron pairs.
Formal Charge and Bonding Patterns
Formal charge helps determine the bonding patterns within a molecule. It aids in identifying the presence of formal charges on individual atoms and their impact on the bonding behavior.
Formal Charge and Periodic Trends
Formal charge can be used to analyze and interpret periodic trends. By examining formal charges across a group or period in the periodic table, scientists can draw conclusions about electron distribution patterns.
The Relationship between Formal Charge and Molecular Energy
Formal charge has a direct impact on the energy of a molecule. A balanced distribution of electrons leads to a more stable and lower energy state.
Limitations of Formal Charge
While formal charge is a valuable tool, it has limitations. It assumes an equal sharing of bonding electrons and does not account for resonance effects or the delocalization of electrons.
In conclusion, the 18 captivating facts about formal charge provide a deeper understanding of this essential concept in chemistry. Formal charge plays a significant role in understanding the stability, reactivity, and structural properties of molecules. By considering formal charges, chemists can unveil the mysteries of molecular behavior and predict the outcomes of chemical reactions.
Conclusion
Formal charge is a fundamental concept in chemistry that helps us understand the distribution of electrons in molecules. By calculating the formal charge of each atom, we can determine the stability and reactivity of a compound. In this article, we have explored 18 captivating facts about formal charge. We have learned that formal charge can be positive, negative, or zero, and that it allows us to identify the most stable resonance structures. We have also discovered that formal charge plays a crucial role in the study of molecular geometry and the prediction of chemical reactions. Understanding formal charge empowers chemists to make informed decisions and unravel the intricacies of chemical compounds. So, the next time you encounter the concept of formal charge, remember these captivating facts and delve even further into the fascinating world of chemistry.
FAQs
1. What is formal charge?
Formal charge is a calculation used in chemistry to determine the number of valence electrons an atom possesses in a molecule. It helps us understand the distribution of electrons and predict the stability and reactivity of compounds.
2. How is formal charge calculated?
Formal charge is calculated by subtracting the number of lone pair electrons and half the number of bonding electrons from the number of valence electrons an atom has in its neutral state.
3. What does a positive formal charge indicate?
A positive formal charge indicates an atom has lost electrons and has fewer electrons than its neutral state. It suggests that the atom is electron-deficient and may be more reactive.
4. What does a negative formal charge indicate?
A negative formal charge indicates an atom has gained electrons and has more electrons than its neutral state. It suggests that the atom has an excess of electrons and may be more stable.
5. How does formal charge affect molecular geometry?
Formal charge affects molecular geometry by influencing the arrangement of atoms and the bond angles in a molecule. It helps determine the most stable structure and predict the shape of a compound.
6. Can formal charge predict chemical reactions?
Yes, formal charge can provide insights into the stability and reactivity of compounds, allowing us to predict and understand chemical reactions. It helps identify potential sites of electron transfer and participation in chemical reactions.
7. What is the significance of formal charge in resonance structures?
Formal charge helps us identify the most stable resonance structures of a compound. By minimizing the formal charges on atoms and distributing electrons optimally, we can determine the most accurate representation of a molecule’s structure.
8. How is formal charge useful in organic chemistry?
Formal charge is highly useful in organic chemistry as it provides vital information about the stability and reactivity of organic compounds. It guides us in predicting electron flow in organic reactions and understanding the behavior of functional groups.
9. Are there any exceptions to the rules of formal charge?
While formal charge is generally a reliable tool, there can be exceptions in certain scenarios. For example, resonance structures and electron delocalization can influence the formal charges of atoms and complicate the analysis.
10. Where can I learn more about formal charge?
You can learn more about formal charge in chemistry textbooks, online educational resources, and through guided practical experiments in chemistry laboratories. Consulting with a chemistry instructor or tutor can also provide in-depth insights into the concept.
Mastering formal charge opens doors to understanding molecular structure, bonding patterns, and reactivity. Valence electrons play a crucial role in determining formal charges, while oxidation states provide complementary insights into redox processes. Applying formal charge concepts to Lewis structures enhances your ability to predict molecular geometry and stability. Dive deeper into these fascinating topics and expand your chemistry knowledge.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
