Molecular orbital diagrams play a crucial role in the understanding of chemical bonding and the properties of molecules. These diagrams provide a visual representation of the arrangement of electrons in molecules, shedding light on their stability and reactivity. Whether you’re a chemistry enthusiast or a student learning the basics of molecular structure, exploring the fascinating world of molecular orbital diagrams is a must. In this article, we will delve into 17 astounding facts about molecular orbital diagrams that will deepen your understanding and appreciation of their significance in the field of chemistry. From the concept of hybridization to the interpretation of bonding and antibonding molecular orbitals, get ready to uncover intriguing insights that will make you see chemical bonding in a whole new light. So, let’s dive into the captivating world of molecular orbital diagrams and discover the wonders they reveal!
Key Takeaways:
- Molecular orbital diagrams show how atoms bond to form molecules and predict their stability and strength. They help us understand chemical reactions and the properties of different substances.
- By using molecular orbital diagrams, scientists can predict and explain the behavior of molecules, helping to develop new materials and understand the world on a molecular level.
Molecular orbital diagram depicts the arrangement of electrons in a molecule.
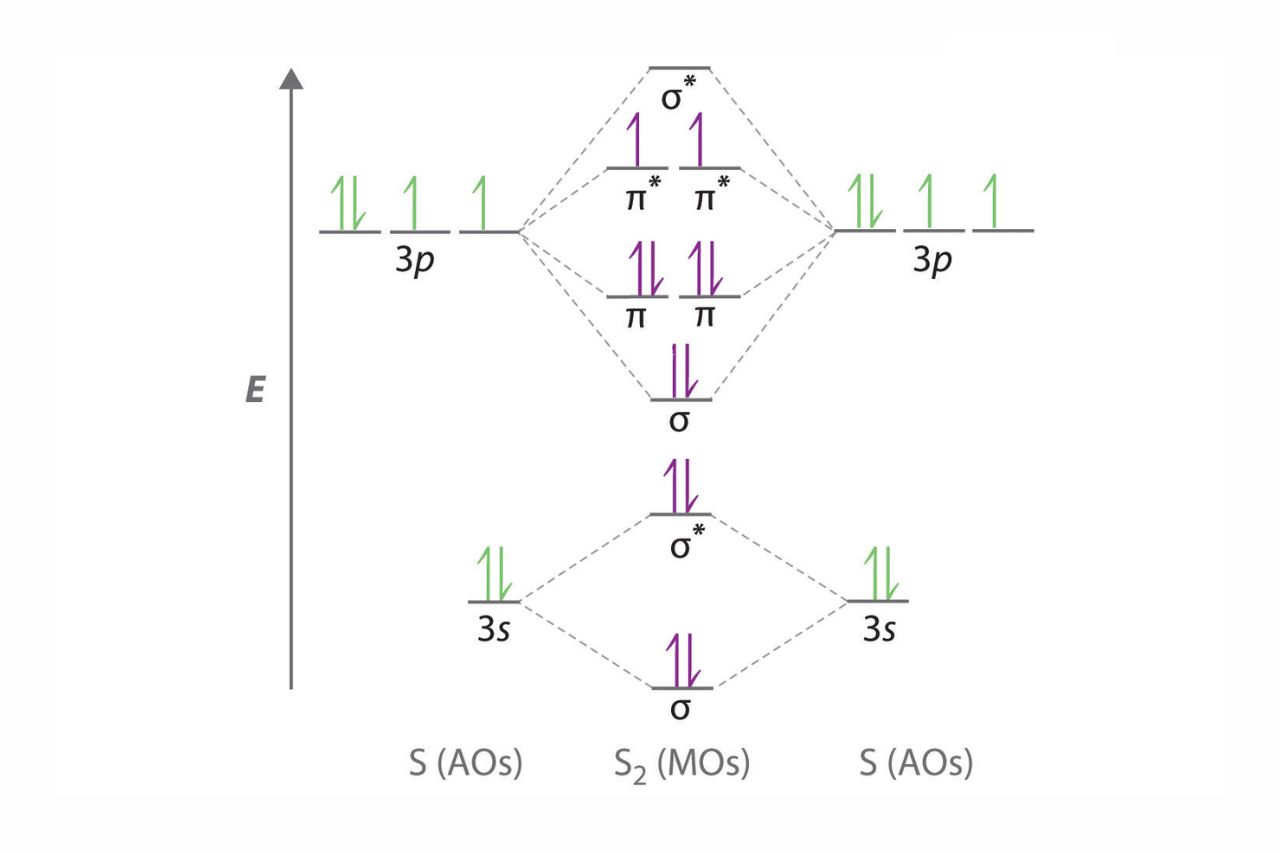
A molecular orbital diagram provides a visual representation of how the atomic orbitals of different atoms combine to form molecular orbitals. It helps in understanding the bonding and properties of molecules.
Molecular orbital diagrams show bonding and antibonding orbitals.
In a molecular orbital diagram, bonding orbitals are represented by lower-energy levels, while antibonding orbitals are shown at higher energy levels. The interaction between these orbitals determines the stability and strength of the bond.
The number of molecular orbitals is equal to the number of atomic orbitals used.
When atomic orbitals combine to form molecular orbitals, the total number of molecular orbitals generated is equal to the number of atomic orbitals involved. This is known as the conservation of orbitals principle.
Molecular orbital diagrams can predict the bond order of a molecule.
By analyzing the molecular orbital diagram, one can determine the bond order, which indicates the stability and strength of the bond. Higher bond order signifies a stronger bond.
Electrons in molecular orbitals follow the Aufbau principle.
The Aufbau principle states that electrons occupy the lowest energy molecular orbitals first before filling higher energy levels. This helps in understanding the electronic configuration of molecules.
The molecular orbital diagram of diatomic molecules consists of sigma and pi orbitals.
In diatomic molecules, the molecular orbital diagram includes sigma (?) and pi (?) orbitals. Sigma orbitals have cylindrical symmetry along the molecular axis, while pi orbitals are perpendicular to the molecular axis.
Molecular orbital diagrams can explain the concept of bond length and bond strength.
The arrangement and overlapping of atomic orbitals in a molecular orbital diagram can provide insights into the bond length, which is the distance between the nuclei of bonded atoms, and bond strength, which is the energy required to break the bond.
The stability of a molecule can be determined from the molecular orbital diagram.
A stable molecule has a lower energy state compared to its constituent atoms. By evaluating the energy levels of molecular orbitals in the diagram, one can assess the stability of the molecule.
Molecular orbital diagrams are used in predicting and explaining chemical reactivity.
Chemical reactions involve the breaking and forming of bonds. Molecular orbital diagrams aid in understanding the stability and reactivity of molecules, helping to predict the likelihood of certain reactions.
Molecular orbital diagrams are essential in understanding the concept of hybridization.
Hybridization involves the mixing of atomic orbitals to form hybrid orbitals, which in turn influence the molecular orbital diagram and bonding characteristics of a molecule. It plays a significant role in explaining molecular geometry.
Molecular orbital diagrams can illustrate the concepts of paramagnetism and diamagnetism.
Paramagnetic substances have unpaired electrons and are attracted to a magnetic field, whereas diamagnetic substances have all paired electrons and are weakly repelled by a magnetic field. Molecular orbital diagrams can show the presence or absence of unpaired electrons, thereby explaining the magnetic properties of molecules.
The shape of the molecular orbital diagram depends on the symmetry of the molecule.
The symmetry of a molecule influences the arrangement of atomic orbitals and the resulting molecular orbital diagram. It determines the types and distribution of bonding and antibonding orbitals.
Molecular orbital diagrams help explain the phenomenon of resonance.
Resonance occurs when a molecule can be represented by different electron distribution patterns. Molecular orbital diagrams can depict the delocalization of electrons, which is fundamental to understanding resonance structures.
Molecular orbital diagrams are used in studying conjugated systems.
Conjugated systems are arrangements of alternating single and multiple bonds. Molecular orbital diagrams are crucial in analyzing the stability and electronic properties of conjugated molecules, such as those found in organic chemistry.
Molecular orbital diagrams can explain the phenomenon of aromaticity.
Aromatic molecules possess a high degree of stability due to the delocalization of pi electrons. Molecular orbital diagrams aid in understanding the unique bonding characteristics of aromatic compounds.
Molecular orbital diagrams can be constructed using various computational methods.
Modern computational methods, such as the Hartree-Fock method or density functional theory (DFT), can be employed to calculate molecular orbital energies and construct molecular orbital diagrams.
Molecular orbital diagrams are widely used in theoretical and computational chemistry.
Molecular orbital diagrams serve as a fundamental tool in theoretical and computational chemistry, helping researchers to predict and explain the properties, behavior, and interactions of molecules.
Conclusion
The molecular orbital diagram is a fascinating aspect of chemistry that helps us understand the behavior and properties of molecules. Through this diagram, we can visualize the distribution of electrons in different molecular orbitals, which in turn influences their bonding and reactivity.
By applying principles of quantum mechanics, scientists have been able to develop molecular orbital theory, which provides a deeper understanding of molecular structures and their properties. It allows us to predict and explain phenomena such as bond strength, polarity, and molecular stability.
Exploring the molecular orbital diagram opens up a world of possibilities in fields such as drug discovery, material science, and environmental chemistry. As we continue to delve deeper into the mysteries of molecular structures, our knowledge and appreciation of the chemical world grow.
So next time you encounter a molecular orbital diagram, take a moment to appreciate the astounding complexity and beauty it represents.
FAQs
Q: What is a molecular orbital diagram?
A: A molecular orbital diagram is a visual representation of the arrangement of electrons in different molecular orbitals, which are formed by the combination of atomic orbitals.
Q: How is a molecular orbital diagram created?
A: A molecular orbital diagram is created by combining the atomic orbitals of the atoms involved in a molecule using the principles of quantum mechanics. The resulting molecular orbitals are arranged in energy levels, with lower energy levels closer to the nucleus.
Q: What information can be obtained from a molecular orbital diagram?
A: A molecular orbital diagram provides information about the bonding and antibonding interactions between atoms in a molecule. It helps in understanding the stability, bond strength, and reactivity of the molecule.
Q: How does a molecular orbital diagram affect molecular properties?
A: The distribution of electrons in different molecular orbitals influences properties such as bond length, bond strength, molecular polarity, and stability. It determines the overall shape and characteristics of a molecule.
Q: Why is understanding molecular orbital diagrams important?
A: Understanding molecular orbital diagrams is crucial for predicting and explaining chemical reactions, designing new compounds, and studying the behavior of molecules. It provides insights into the electronic structure of molecules and how it relates to their properties.
Molecular orbital diagrams offer a fascinating glimpse into the world of chemistry. But there's so much more to explore! Dive into the captivating realm of theoretical chemistry, where cutting-edge concepts and ideas shape our understanding of the chemical universe. Unravel the mysteries of chemical bonding, the very force that holds molecules together. And don't forget to expand your knowledge of molecular orbital theory, the foundation upon which these diagrams are built. Each topic is a thrilling journey waiting to be discovered, so why not embark on your next chemistry adventure today?
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
