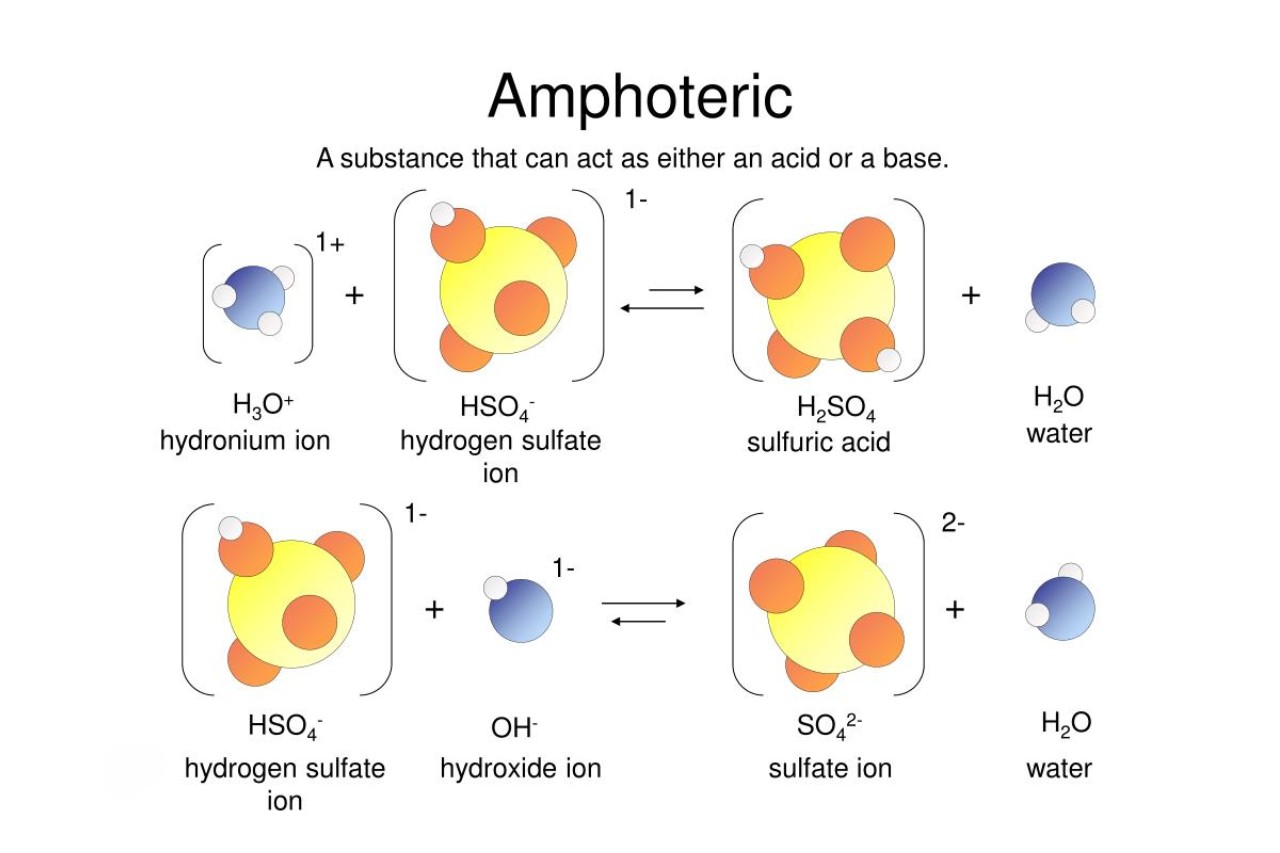
When it comes to chemistry, there are certain substances that truly leave us in awe of their properties. One such substance is known as an amphoteric substance. These unique compounds possess the ability to both donate and accept protons, making them incredibly versatile and fascinating.
In this article, we will delve into the world of amphoteric substances and explore 20 mind-blowing facts about them. From their role in buffering solutions to their significance in biological systems, amphoteric compounds have a multitude of applications and play a vital role in the world of chemistry.
So, get ready to expand your chemical knowledge as we uncover some intriguing details about these extraordinary substances.
Key Takeaways:
- Amphoteric substances can act as both acids and bases, making them versatile and highly reactive in chemical reactions. They play a crucial role in maintaining stable pH environments in buffer solutions.
- Understanding amphoteric substances is essential in chemistry education, as they are involved in pH regulation in living organisms and have applications in various industries like pharmaceuticals and cosmetics.
Amphoteric substances can act as both acids and bases.
Unlike most substances that are either acidic or basic, amphoteric substances have the unique ability to exhibit both acid and base properties depending on the reaction they are involved in.
Amphoteric substances can react with both acids and bases.
Due to their versatile nature, amphoteric substances can react with both acidic and basic solutions, making them highly reactive compounds in chemical reactions.
A classic example of an amphoteric substance is water.
Water is the most well-known amphoteric substance. It can act as both an acid and a base, depending on the substances with which it reacts.
Amphoteric substances can form salts.
When an amphoteric substance reacts with an acid or a base, it can form a salt by neutralizing the acidic or basic properties of the substance with which it reacts.
Zinc oxide is another common example of an amphoteric substance.
Zinc oxide, often used in sunscreens and skincare products, exhibits amphoteric properties by reacting with both acids and bases.
Amphoteric substances play a crucial role in buffer solutions.
Buffer solutions contain amphoteric substances that can resist changes in pH by accepting or donating protons, helping to maintain a stable pH environment.
Many metal oxides exhibit amphoteric behavior.
Metal oxides, such as aluminum oxide and chromium oxide, can behave as both acids and bases in certain reactions, depending on the conditions.
Amphoteric substances can be found in nature.
Natural substances like proteins and amino acids have amphoteric properties, allowing them to participate in a variety of chemical reactions.
Amphoteric substances can act as catalysts.
Due to their ability to interact with both acids and bases, amphoteric substances are often used as catalysts to facilitate chemical reactions.
The pH of amphoteric substances can change depending on the environment.
The pH of amphoteric substances can shift towards the acidic or basic side, depending on the pH of the surrounding solution and the nature of the reacting species.
Amphoteric phenomena are observed in amphiphilic molecules.
Amphiphilic molecules, like soap, possess both hydrophobic and hydrophilic properties, making them amphoteric substances.
Amphoteric substances have applications in various industries.
Due to their versatility, amphoteric substances find applications in industries such as pharmaceuticals, cosmetics, and agriculture.
Amphoteric substances can undergo hydrolysis reactions.
In hydrolysis reactions, amphoteric substances can react with water to form acidic or basic solutions depending on the nature of the substance.
The amphoteric nature of substances can be determined through pH measurements.
By measuring the pH of a substance and observing whether it exhibits acidic or basic properties, its amphoteric nature can be determined.
Some amphoteric substances have medicinal properties.
Compounds with amphoteric properties are utilized in the development of medicines for their ability to interact with different types of molecules in the body.
Amphoteric substances are important in analytical chemistry.
Their ability to react with both acids and bases makes amphoteric substances valuable in the field of analytical chemistry, where precise measurements and reactions are crucial.
Amphoteric substances can exhibit amphoterism in specific temperature ranges.
The amphoteric behavior of certain substances may be observed only within specific temperature ranges, highlighting the importance of controlling reaction conditions.
Amphoteric substances are involved in pH regulation in living organisms.
Biological systems rely on amphoteric substances to maintain proper pH levels and enable crucial biochemical reactions.
The amphoteric behavior of substances is influenced by molecular structure.
The arrangement and composition of atoms in a molecule determine whether it will exhibit amphoteric properties.
Understanding amphoteric substances is essential in chemistry education.
Studying amphoteric substances enhances our understanding of chemical reactions, acid-base concepts, and their applications in various scientific disciplines.
These 20 mind-blowing facts about amphoteric substances showcase the versatility and significance of these compounds in chemistry and various industries. From their ability to act as acids and bases to their applications in buffer solutions and catalysis, understanding amphoteric substances is crucial for anyone interested in the fascinating world of chemistry.
Conclusion
In conclusion, amphoteric substances are truly fascinating and versatile in their properties. They possess the ability to act as either acids or bases depending on the conditions they are present in. This unique characteristic allows them to participate in a wide array of chemical reactions.From their importance in industries such as pharmaceuticals and cleaning agents to their role in biological systems, amphoteric substances play a crucial role in our everyday lives. Their ability to react with both acidic and basic substances make them incredibly valuable in various scientific applications.Understanding the behavior and properties of amphoteric substances is essential for chemists and researchers working in the field of chemistry. By studying these substances, we can uncover new insights into chemical reactions and pave the way for innovative technologies and advancements.So, the next time you encounter an amphoteric substance, take a moment to appreciate its remarkable properties and the crucial role it plays in the world of chemistry.
FAQs
Q: What is an amphoteric substance?
A: An amphoteric substance is a chemical compound that has the ability to react as both an acid and a base, depending on the conditions. It can donate or accept protons in a reaction.
Q: Can you give some examples of amphoteric substances?
A: Yes, some examples of amphoteric substances include water (H2O), amino acids, aluminum hydroxide (Al(OH)3), and zinc oxide (ZnO).
Q: How do amphoteric substances behave in acidic conditions?
A: In acidic conditions, amphoteric substances tend to act as bases by accepting protons. They can neutralize the excess acid by reacting with it.
Q: And how do they behave in basic conditions?
A: In basic conditions, amphoteric substances behave as acids by donating protons. They can neutralize the excess base by reacting with it.
Q: What are the applications of amphoteric substances?
A: Amphoteric substances are widely used in various industries. For example, they are used as cleaning agents, additives in pharmaceuticals, and even in sunscreen formulations.
Q: Can amphoteric substances exist in both a solid and liquid state?
A: Yes, amphoteric substances can exist in both solid and liquid states. The state of the substance depends on factors such as temperature and pressure.
Q: Are all substances amphoteric?
A: No, not all substances are amphoteric. Amphoteric substances are specific chemical compounds that possess the ability to react as both acids and bases.
Amphoteric substances are truly fascinating, but there's more to explore in the world of chemistry. Dive into the enigmatic realm of isomerism and uncover the astounding chemical properties that make these compounds so unique. Acids and bases may seem simple, but they hold a treasure trove of fun facts waiting to be discovered. For those captivated by amphoteric substances, a deeper understanding of amphoterism will unveil even more intriguing insights. Embark on a journey through these captivating topics and expand your knowledge of the incredible world of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
