Molality is a fundamental concept in chemistry that plays a crucial role in understanding the behavior of chemical solutions. It refers to the concentration of a solute in a solvent and is expressed as the number of moles of solute per kilogram of solvent. Molality is a powerful tool for measuring and predicting various properties of solutions, such as boiling point elevation, freezing point depression, and osmotic pressure.
In this article, we will delve into the fascinating world of molality and explore 18 intriguing facts that will deepen your understanding of this important concept. From its origins and significance to its practical applications and effects on physical properties, we will uncover the many facets of molality that make it an essential part of studying and applying chemistry.
Key Takeaways:
- Molality measures solution concentration in moles per kilogram of solvent. It’s crucial for understanding freezing point depression, boiling point elevation, and other colligative properties in chemistry experiments.
- Unlike molarity, molality is temperature-independent, making it more accurate for scientific applications. It’s essential in chemical reactions, industrial processes, and even in determining food safety in food science.
The Concept of Molality
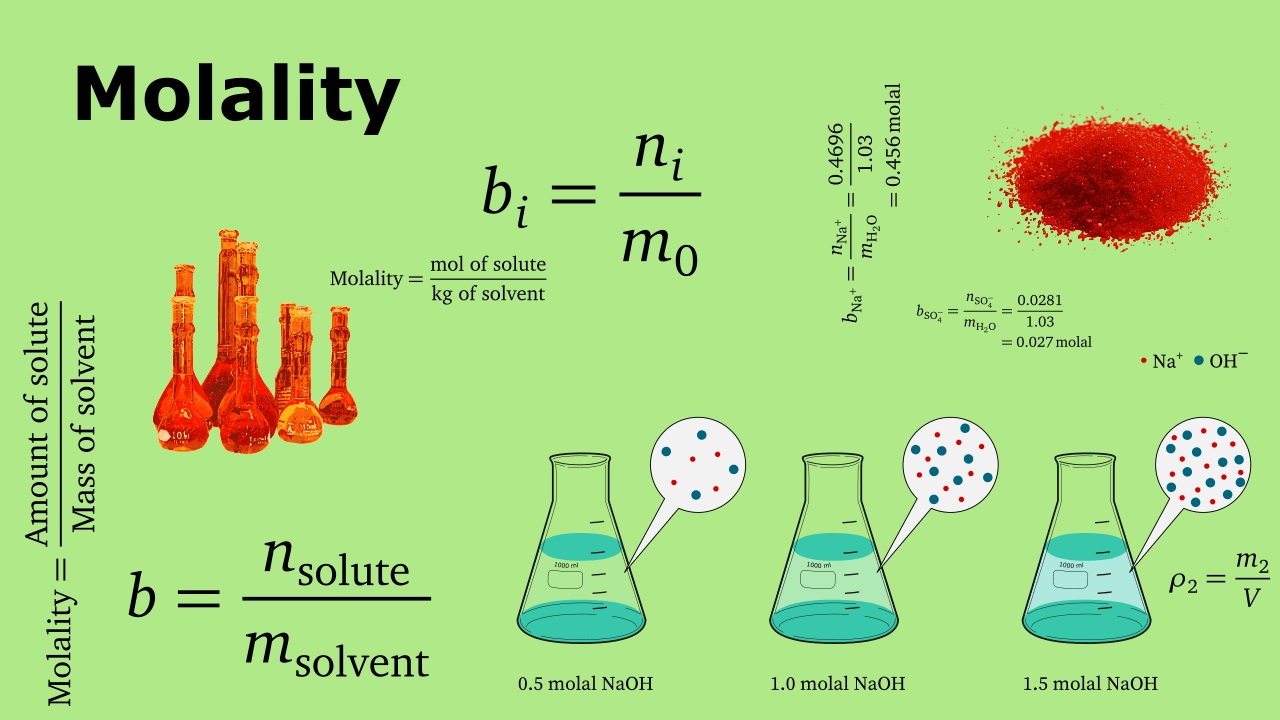
Molality is a fundamental concept in chemistry that measures the concentration of a solution in terms of the number of moles of solute per kilogram of solvent. It is denoted by the symbol “m” and is often used in contrast to molarity, which measures concentration in terms of number of moles of solute per liter of solution. Unlike molarity, molality is temperature independent, making it a more accurate way to express concentration in certain scientific applications.
Molality Calculation
To calculate molality, the following formula is used:
Molality (m) = Moles of Solute / Mass of Solvent (in kilograms)
The Relationship Between Molality and Freezing Point Depression
One of the key applications of molality is in explaining the phenomenon of freezing point depression. When a solute is added to a solvent, it lowers the freezing point of the solvent. The extent of freezing point depression is directly proportional to the molality of the solute in the solution.
Molal Boiling Point Elevation
Similar to freezing point depression, molality also plays a role in boiling point elevation. When a solute is dissolved in a solvent, it raises the boiling point of the solvent. The increase in boiling point is directly proportional to the molality of the solute.
Molality in Colligative Properties
Molality is involved in various colligative properties, which depend on the number of particles present in a solution rather than the specific nature of the particles. These properties include vapor pressure lowering, osmotic pressure, and boiling point elevation.
The Importance of Molality in Laboratory Experiments
Molality is crucial in laboratory experiments as it allows scientists to accurately measure and adjust the concentration of solutions. This is especially important in fields such as analytical chemistry, where precise control of solute concentrations is vital for accurate analysis.
Molality and Chemical Reactions
In chemical reactions, molality is used to determine the stoichiometry of the reaction by determining the amount of reactants and products present in a specified amount of solvent.
Temperature Independence of Molality
Unlike molarity, which is temperature-dependent, molality remains constant regardless of changes in temperature. This makes it particularly useful in thermodynamic calculations and experiments.
Molality and Solubility
Molality also plays a role in determining the solubility of a solute in a solvent. The solubility of a substance is often expressed in terms of grams of solute per kilogram of solvent, which relates to the molality of the solution.
Molality Versus Molarity
While molality and molarity both measure concentration, they differ in their units of measurement. Molality is expressed in units of moles per kilogram (mol/kg), while molarity is expressed in moles per liter (mol/L).
The Role of Molality in Industrial Processes
Molality is utilized in various industrial processes such as chemical manufacturing, pharmaceutical production, and food processing. Knowing the molality of a solution allows for precise control and optimization of these processes.
Molality and Biological Systems
Understanding molality is essential in biological systems, as it helps in determining the concentration of solutes in various biological fluids, such as blood, urine, and intracellular fluids. This information is crucial in medical diagnostics and research.
Molality and Colligative Properties of Solutions
Colligative properties, which depend on the concentration of solutes, are directly related to molality. These properties include the lowering of vapor pressure, the elevation of boiling point, osmotic pressure, and the lowering of freezing point.
Molality and Osmosis
Osmosis, the movement of solvent molecules across a semipermeable membrane, is influenced by the molality of the solute. Changes in molality can impact the direction and rate of osmotic flow.
Molality and Electrolytic Solutions
Molality plays a crucial role in determining the behavior of electrolytic solutions. Electrolytes dissociate into ions in solution, and their concentration affects various electrochemical processes, such as conductivity and electrode potential.
Molality in Environmental Studies
Molality is used in environmental studies to determine the concentration of pollutants in water bodies and other environmental samples. It helps in assessing the impact of contaminants on ecosystems and human health.
Molality in Food Science
Molality is important in the field of food science as it allows for accurate measurements of solute concentrations in food products. This knowledge is crucial for developing recipes, ensuring food safety, and maintaining consistent product quality.
Molality and the Development of New Materials
Molality is a critical factor in the synthesis and development of new materials. Understanding the concentration of reactants and solvents is essential for controlling the properties and characteristics of the final product.
Conclusion
In conclusion, molality is a fascinating concept in chemistry that plays a crucial role in understanding the concentration of solutes in a solution. It provides a more accurate representation of concentration than other measures such as molarity, especially in situations involving temperature changes or when dealing with mixtures that are not predominantly water-based.By incorporating molality into our understanding of solutions, we can better comprehend various phenomena in chemistry, such as colligative properties and the behavior of electrolytes. Molality allows us to make precise calculations and predictions, leading to advancements in fields ranging from pharmaceuticals to environmental science.Understanding the intricate details surrounding molality can be challenging, but with practice and patience, anyone can grasp its significance in the world of chemistry. So the next time you encounter a solution, remember to consider molality and delve deeper into its intriguing effects.
FAQs
Q: What is molality?
A: Molality is a measure of the concentration of solute in a solution, expressed as the number of moles of solute per kilogram of solvent.
Q: How is molality different from molarity?
A: While molality measures the concentration in terms of moles of solute per kilogram of solvent, molarity measures the concentration in terms of moles of solute per liter of solution.
Q: Why is molality important in chemistry?
A: Molality is important because it allows for accurate calculations and predictions, especially in situations where temperature changes or non-aqueous solvents are involved.
Q: How do you calculate molality?
A: Molality is calculated by dividing the moles of solute by the mass of the solvent (in kilograms). The equation is: molality (m) = moles of solute / mass of solvent (in kg).
Q: What are some practical applications of molality?
A: Molality is used in various fields, such as pharmaceutical manufacturing, food science, environmental science, and chemical engineering, to accurately measure and control concentrations of solutes in solutions.
Q: Can molality change with temperature?
A: No, molality does not change with temperature. Unlike molarity, which is temperature-dependent due to changes in volume, molality remains constant as it is based on the mass of the solvent.
Molality's fascinating insights barely scratch the surface of chemistry's wonders. Dive deeper into stoichiometry's enigmatic calculations, uncover concentration's captivating influence on reactions, or explore chemistry's vast landscape of knowledge. Whether you're a budding scientist or simply curious, these topics offer endless opportunities for discovery and understanding.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
