Osmotic pressure is a fascinating concept in the field of chemistry that plays a crucial role in various biological and chemical processes. It refers to the pressure exerted by a solvent when it is separated by a semipermeable membrane from a solution containing solute particles. Osmotic pressure is not only a fundamental concept in chemistry but also holds great importance in industries like pharmaceuticals, agriculture, and food processing. Understanding osmotic pressure is essential for comprehending the process of osmosis, which is the movement of solvent molecules from a region of lower solute concentration to a region of higher solute concentration across a membrane. In this article, we will explore 17 extraordinary facts about osmotic pressure, shedding light on its definition, significance, applications, and some intriguing real-life examples. So, let’s dive deep into the fascinating world of osmotic pressure!
Key Takeaways:
- Osmotic pressure is the movement of solvent molecules to balance solute concentration, crucial for biological systems, food preservation, and even medication delivery.
- Understanding osmotic pressure helps us unlock new possibilities in technology and industry, from desalination to drug delivery systems.
What Is Osmotic Pressure?
Osmotic pressure is the pressure exerted by a solvent when it is separated by a semipermeable membrane from a solution containing a solute. This phenomenon is a result of the movement of solvent molecules from an area of lower solute concentration to an area of higher solute concentration, in order to achieve equilibrium.
Osmotic Pressure and Biological Systems
Osmotic pressure plays a crucial role in biological systems, such as the movement of water and nutrients across cell membranes. It helps regulate the balance of fluids inside and outside of cells, ensuring proper cell function.
Colligative Property
Osmotic pressure is a colligative property, meaning it depends solely on the concentration of solute particles, regardless of their size or identity. This makes it a valuable tool for determining molecular weights and studying the behavior of solutions.
Van’t Hoff Equation
The Van’t Hoff equation is commonly used to calculate osmotic pressure. It states that osmotic pressure is directly proportional to the molarity of the solute and the absolute temperature.
Role in Reverse Osmosis
Osmotic pressure is the driving force behind reverse osmosis, a process used for desalination and water purification. By applying pressure greater than the osmotic pressure, water is forced through a semipermeable membrane, leaving behind impurities.
Osmosis and Plant Cells
Osmosis plays a vital role in maintaining the turgidity of plant cells. When a plant cell is placed in a hypotonic solution (lower solute concentration), water moves into the cell, causing it to swell and become turgid.
Osmotic Pressure and Kidney Function
The kidneys regulate osmotic pressure in the body by selectively reabsorbing water and solutes from the filtrate. This process ensures that essential nutrients are retained while excess waste products are eliminated.
Osmotic Pressure and Preservation
Osmotic pressure has been used for centuries as a method of food preservation. Placing food in a concentrated solution, such as salt or sugar, draws out water from microorganisms, inhibiting their growth and prolonging the shelf life of the food.
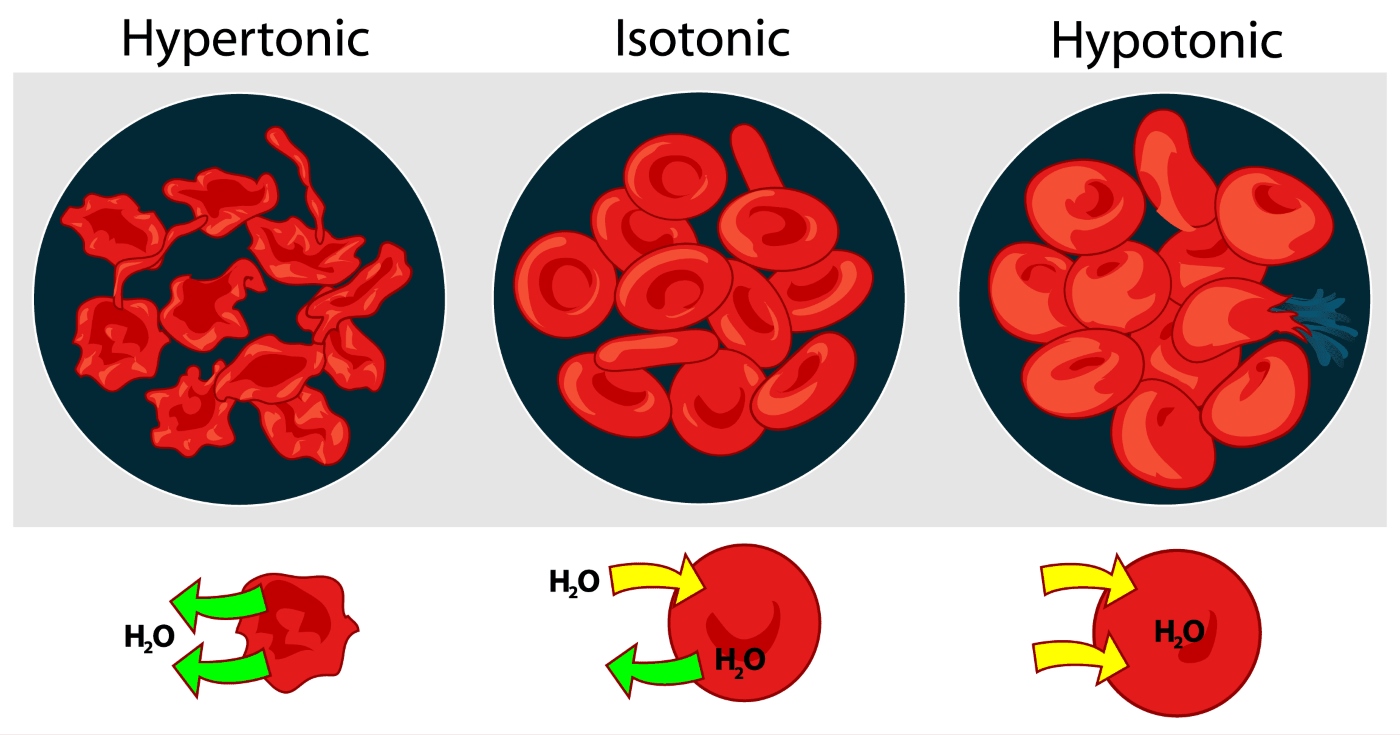
Osmotic Pressure in Red Blood Cells
Red blood cells must maintain a delicate balance of osmotic pressure to function properly. If the surrounding solution is too hypotonic, water can enter the cells and cause them to burst. Conversely, if the solution is too hypertonic, water leaves the cells, leading to shrinkage.
Importance in Industrial Processes
Osmotic pressure is utilized in various industrial processes, including the production of pharmaceuticals, wastewater treatment, and the manufacturing of electronic components. It helps separate solvents from impurities and facilitates the purification of materials.
Osmotic Pressure in Plant Root Absorption
Through osmotic pressure, plants absorb water and nutrients from the soil. The lower solute concentration inside the root cells creates a gradient that allows water to enter through osmosis, supporting plant growth and development.
Osmotic Pressure and Medication Delivery
Osmotic pressure has been harnessed in drug delivery systems. By encapsulating medications in osmotic pumps, controlled release of the drug can be achieved, ensuring a constant and steady supply over an extended period of time.
Osmosis and Aquatic Organisms
Aquatic organisms, such as fish, face osmotic challenges in maintaining the right balance of salt and water in their bodies. They have specialized mechanisms, such as gills and kidneys, to regulate osmotic pressure and survive in different environments.
Osmotic Pressure and Cell Lysis
If a cell is placed in a hypotonic solution, where the external solute concentration is lower than the internal solute concentration, excess water can enter the cell. This can lead to cell lysis, causing the cell to burst.
Osmotic Pressure and Food Industry
Osmotic pressure plays an essential role in food processing, particularly in the production of dried fruits. Immersion in hypertonic solutions causes water to leave the fruits, preserving their flavor, texture, and nutritional value.
Osmotic Pressure and Environmental Engineering
In environmental engineering, osmotic pressure is a key factor in desalination processes, such as forward osmosis and pressure-retarded osmosis. These methods harness osmotic pressure gradients to generate clean drinking water.
Osmotic Pressure and Chemical Separation
Osmotic pressure can be employed to separate different chemical components. By exploiting the differences in solute concentrations, osmotic processes can selectively extract specific compounds, aiding in separation and purification.
These 17 extraordinary facts about osmotic pressure highlight its significance in various fields, from biology to industry. Understanding osmotic pressure not only expands our knowledge of natural processes but also unlocks new possibilities for technological advancements.
Conclusion
In conclusion, osmotic pressure is a fascinating phenomenon that plays a vital role in various biological and chemical processes. Understanding the concept of osmotic pressure not only helps us comprehend the behavior of solutions but also enables us to explain a wide range of phenomena in nature. From the ability of plants to absorb water through their roots to the mechanism of dialysis in medical treatments, osmotic pressure truly has extraordinary implications.The study of osmotic pressure has paved the way for advancements in fields such as chemistry, biology, and medicine. Researchers and scientists continue to explore its intricacies, unraveling its secrets and finding new applications.By delving into the 17 extraordinary facts about osmotic pressure, we have gained a deeper appreciation for its significance. Whether it be osmosis, colligative properties, or the role of membranes, osmotic pressure remains an intriguing subject that blends the principles of physics and chemistry. So, the next time you encounter a solution, remember that there is more to it than meets the eye, and osmotic pressure is at play.
FAQs
Q: What is osmotic pressure?
A: Osmotic pressure is the pressure exerted by a solvent when it passes through a semipermeable membrane to equalize the concentration of solute on either side.
Q: Why is osmotic pressure important?
A: Osmotic pressure is important as it allows for the movement of water and essential molecules across cell membranes, maintaining cell function and homeostasis.
Q: How is osmotic pressure calculated?
A: Osmotic pressure can be calculated using the equation ? = cRT, where ? is osmotic pressure, c is the concentration of the solute, R is the ideal gas constant, and T is the temperature in Kelvin.
Q: What is the significance of osmotic pressure in plants?
A: Osmotic pressure plays a crucial role in water absorption by plant roots. It helps to maintain turgor pressure, which supports the structure of plant cells and enables the transport of nutrients.
Q: Can osmotic pressure be used in medical treatments?
A: Yes, osmotic pressure is utilized in medical treatments such as dialysis. By creating a concentration gradient, osmotic pressure facilitates the removal of waste products from the bloodstream.
Q: Are there any practical applications of osmotic pressure?
A: Osmotic pressure is applied in various industries, including water purification, food preservation, and pharmaceutical manufacturing, to achieve specific desired outcomes.
Osmotic pressure plays a crucial role in various aspects of life, from biological systems to industrial processes. Understanding its principles and applications can help you appreciate the fascinating world of chemistry. If you found these facts about osmotic pressure intriguing, you might also enjoy exploring the concepts of molality and colligative properties. Delve into the mysteries of molality and uncover its significance in chemical solutions. Additionally, prepare to be amazed by the unbelievable facts surrounding colligative properties and how they influence the behavior of matter. Continue your journey of discovery and expand your knowledge of these captivating chemical phenomena.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
