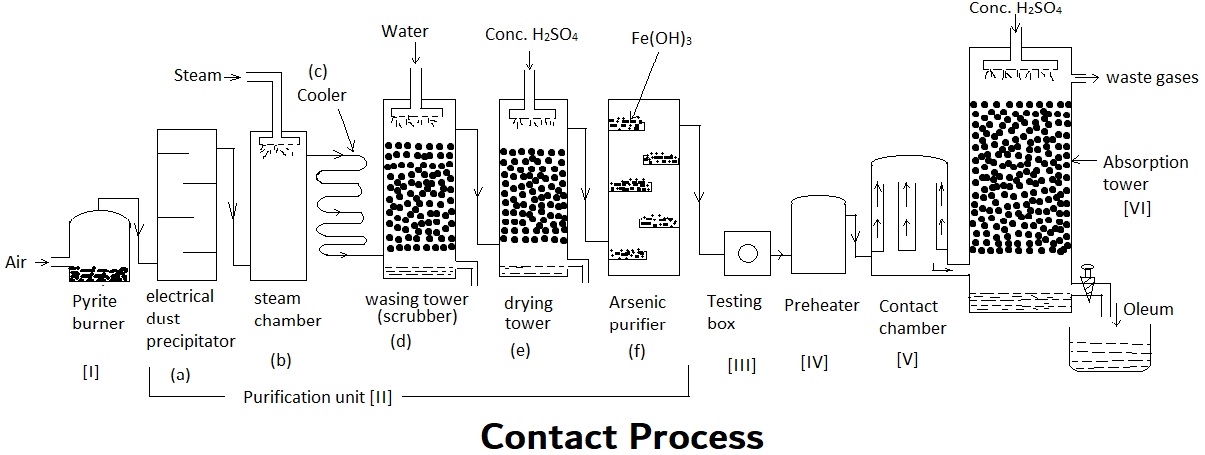
Chemistry is a captivating branch of science that unveils the fascinating secrets of the elements and compounds that make up our world. One of the most significant processes in the field of chemistry is the Contact Process. This chemical reaction plays a crucial role in the production of sulfuric acid, which is an essential ingredient in many industrial processes.
In this article, we will explore 17 unbelievable facts about the Contact Process, shedding light on the intricacies of this reaction and its impact on various industries. From its historical background and discovery to its mechanism and uses, you will discover the amazing world hidden behind this remarkable chemical process.
Key Takeaways:
- The Contact Process is a crucial method for making sulfuric acid, which is used in many everyday products. It involves high temperatures, a special catalyst, and a reversible reaction.
- The Contact Process has a big impact on the world economy and the environment. It’s important for making things like fertilizers and dyes, but it also needs to be made more eco-friendly.
Easily Convertible Substances
The Contact Process is a chemical process used to produce sulfuric acid, one of the most widely used industrial chemicals worldwide. It involves the oxidation of sulfur dioxide (SO2), which is readily available from the combustion of sulfur-containing fuels such as coal or oil.
The Catalyst
A key component of the Contact Process is the catalyst, which is typically vanadium pentoxide (V2O5). This catalyst helps to speed up the reaction between sulfur dioxide and oxygen, allowing for a more efficient production of sulfur trioxide (SO3), the precursor to sulfuric acid.
High Temperatures
The Contact Process requires high temperatures for the reaction to occur. Typically, the reaction takes place at around 450-500 degrees Celsius. These high temperatures are necessary to provide enough energy for the reaction to proceed at a reasonable rate.
The Role of Oxygen
Oxygen is a vital component in the Contact Process as it acts as an oxidizing agent. During the reaction, oxygen reacts with sulfur dioxide to form sulfur trioxide. This reaction is exothermic, meaning it releases heat as a byproduct.
Efficiency Enhancement
The Contact Process has undergone numerous advancements to improve its efficiency. One notable improvement is the use of catalysts that reduce the activation energy required for the reaction to occur, making it more energy-efficient and cost-effective.
Reversible Reaction
The reaction between sulfur dioxide and oxygen to produce sulfur trioxide is reversible. This means that under certain conditions, sulfur trioxide can react with water vapor to form sulfuric acid, or it can be converted back to sulfur dioxide and oxygen.
Industrial Importance
The Contact Process is of immense industrial importance as sulfuric acid is a vital component in the production of fertilizers, dyes, detergents, and many other chemicals. It also plays a crucial role in various industrial processes, including metal refining and petroleum refining.
Continuous Production
The Contact Process allows for continuous production of sulfuric acid. The reaction takes place in a series of interconnected reactors, ensuring a constant supply of reactants and the continuous removal of the product.
Environmental Considerations
The Contact Process has led to concerns regarding its environmental impact. The combustion of sulfur-containing fuels to produce sulfur dioxide contributes to air pollution and acid rain. Efforts are being made to develop cleaner and more sustainable methods for sulfuric acid production.
Global Dominance
The Contact Process accounts for the majority of global sulfuric acid production. Its efficiency and scalability have contributed to its widespread adoption, making it the preferred method for large-scale production of sulfuric acid.
Advancements in Catalyst Technology
Ongoing research and development in catalyst technology have resulted in the discovery of new catalysts that are more effective and environmentally friendly. These advancements aim to increase the efficiency of the Contact Process while reducing its environmental impact.
Safety Precautions
The Contact Process requires strict adherence to safety protocols due to the high temperatures involved and the potential hazards associated with sulfuric acid. Proper training, protective equipment, and safety measures are essential to ensure a safe working environment.
Energy Consumption
The Contact Process is energy-intensive, requiring significant amounts of heat to maintain the high reaction temperatures. Efforts are being made to optimize the process and minimize energy consumption through the development of more efficient reactors and heat exchange systems.
Monitoring and Control
Continuous monitoring and control systems are employed in the Contact Process to ensure optimal reaction conditions and maintain the desired product quality. These systems help to minimize variations in the process and ensure consistent production.
Global Economic Impact
The production and use of sulfuric acid, facilitated by the Contact Process, have a significant impact on the global economy. It supports various industries and plays a crucial role in driving economic growth and development.
Historical Significance
The development of the Contact Process by the English chemist Peregrine Phillips in 1831 marked a significant milestone in the field of chemical engineering. It revolutionized the production of sulfuric acid and paved the way for numerous industrial applications.
Future Prospects
As the demand for sulfuric acid continues to grow, the Contact Process will likely undergo further advancements to improve its efficiency and sustainability. Research efforts are focused on finding alternative sources of sulfur dioxide and developing more environmentally friendly catalysts.
Conclusion
In conclusion, the contact process is an incredible chemical reaction that plays a crucial role in various industries, especially in the production of sulfuric acid. It involves the oxidation of sulfur dioxide to sulfur trioxide using a catalyst and carefully controlled conditions. The process not only enhances efficiency but also minimizes environmental impact by reducing the emission of harmful gases.By understanding the contact process and its intricate details, we can appreciate the significance of this chemical reaction in our everyday lives. From the production of fertilizers to the manufacturing of dyes and detergents, the contact process enables the production of essential products that contribute to our modern society.As the contact process continues to evolve and improve, scientists and engineers are constantly exploring ways to enhance its efficiency and sustainability. By researching and developing new catalysts, optimizing reaction conditions, and implementing innovative techniques, we can ensure the continued success and relevance of this remarkable chemical process.
FAQs
Q: What is the contact process?
A: The contact process is a chemical reaction used to produce sulfuric acid. It involves the oxidation of sulfur dioxide to sulfur trioxide in the presence of a catalyst.
Q: Why is the contact process important?
A: The contact process is important because it allows for the large-scale production of sulfuric acid, which is a vital raw material used in various industries such as manufacturing fertilizers, dyes, detergents, and more.
Q: What catalyst is used in the contact process?
A: The contact process uses vanadium pentoxide as a catalyst to facilitate the oxidation of sulfur dioxide to sulfur trioxide.
Q: What are the optimal conditions for the contact process?
A: The contact process requires specific conditions such as a temperature range of 400-500°C, a pressure of 1-2 atmospheres, and the presence of a catalyst, typically vanadium pentoxide supported on a silica substrate.
Q: How does the contact process minimize environmental impact?
A: The contact process minimizes environmental impact by reducing the emission of sulfur dioxide gases through careful control of the reaction conditions and the use of efficient catalysts.
The Contact Process is just one fascinating aspect of chemistry. Dive into the world of oxidation reactions, unraveling their mysteries and significance. Catalysis plays a crucial role in countless chemical processes, and exploring its enigmatic facts will leave you in awe. For those curious about the broader field, chemical engineering holds a treasure trove of fascinating insights waiting to be discovered.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
