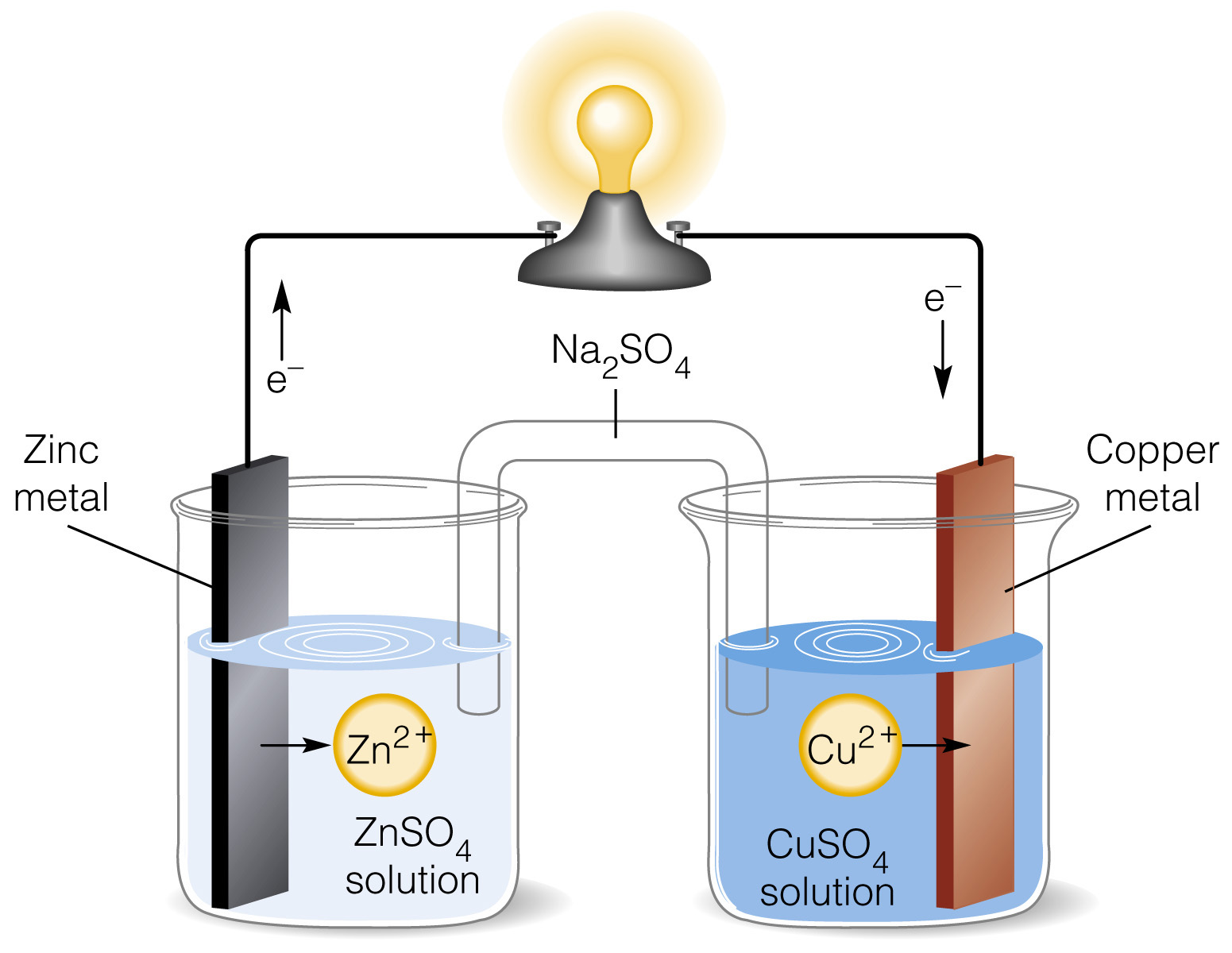
The Daniell cell is a remarkable invention in the field of chemistry, credited to the ingenious work of John Frederic Daniell in the 19th century. This electrochemical cell, also known as the Daniell battery, revolutionized the study and application of electricity. The cell consists of two electrodes – a copper electrode immersed in a copper sulfate solution and a zinc electrode immersed in a zinc sulfate solution – which are connected by a wire. This simple but effective design allows for the conversion of chemical energy into electrical energy.
In this article, we will dive into 15 fascinating facts about the Daniell cell that will not only enhance your understanding of this remarkable invention but also shed light on its wide-ranging applications. From its historical significance to its importance in various fields, these facts will showcase the impact and enduring relevance of the Daniell cell in the world of chemistry and beyond.
Key Takeaways:
- The Daniell Cell, invented in 1836, was a reliable early battery used in telegraphy, with a longer shelf life and stable voltage output.
- Its redox reaction between copper and zinc, longer shelf life, and influence on future battery designs made the Daniell Cell a significant advancement in early electrical technology.
The Daniell Cell was invented by John Frederic Daniell in 1836.
The Daniell Cell, named after its inventor John Frederic Daniell, is an early form of battery that provided a reliable and stable source of electricity in the mid-19th century.
It was widely used in telegraphy.
The Daniell Cell was extensively used in telegraphy systems during its time. Its ability to generate a constant and consistent electrical current made it ideal for transmitting long-distance messages through telegraph lines.
The Daniell Cell consists of two half-cells.
Unlike other batteries of its time, the Daniell Cell is made up of two distinct half-cells: a copper electrode immersed in a copper sulfate solution and a zinc electrode immersed in a zinc sulfate solution. These half-cells are connected by a salt bridge, allowing the flow of ions between them.
It operates based on the redox reaction between copper and zinc.
The Daniell Cell functions through a redox reaction between the copper and zinc electrodes. Zinc atoms oxidize, losing electrons and producing zinc ions, while copper ions from the copper sulfate solution gain these electrons, depositing copper atoms on the copper electrode.
The Daniell Cell has a voltage of around 1.1 volts.
When the Daniell Cell is operating, it typically produces a voltage of approximately 1.1 volts. This stable voltage output was crucial for various applications, including telegraphy and electroplating.
It has a longer shelf life compared to other batteries of its time.
The Daniell Cell had a longer shelf life compared to other batteries available during the 19th century. This was due to the separation of the copper and zinc electrodes, which reduced the self-discharge rate and prolonged its usability.
The Daniell Cell was more efficient than previous battery designs.
Previous battery designs suffered from issues such as polarization and short-circuiting. The Daniell Cell addressed these problems and offered a more efficient and reliable source of electricity.
It played a significant role in advancing electrochemical studies.
The introduction of the Daniell Cell revolutionized the field of electrochemistry. It allowed scientists to study and understand various chemical reactions involving electricity, leading to significant advancements in the field.
The Daniell Cell paved the way for future battery developments.
The Daniell Cell served as a foundation for future advancements in battery technology. Its concept of using different metal electrodes and salt bridge influenced the design of later batteries, including the modern alkaline battery.
It was replaced by more practical and efficient battery designs.
Despite its advantages, the Daniell Cell eventually became obsolete as more practical and efficient battery designs were developed. However, its impact on early electrical technology cannot be undermined.
The Daniell Cell is still used in educational settings.
Although not widely used in practical applications today, the Daniell Cell is still utilized in educational settings to demonstrate fundamental principles of electrochemistry and battery operation.
It provided a foundation for understanding electrical circuits.
The Daniell Cell contributed significantly to the understanding of electrical circuits. Its simple yet effective design helped scientists and engineers grasp the concept of voltage, current, and circuitry.
The Daniell Cell was an important step towards the development of electroplating.
The Daniell Cell played a crucial role in the advancement of electroplating techniques. By providing a stable source of electricity, it enabled the deposition of one metal onto another, leading to the development of electroplating processes for various industries.
It became the basis for the standard reference electrode.
The Daniell Cell’s design and characteristics made it an ideal candidate for the development of the standard reference electrode. This electrode is used as a reference point in many electrochemical measurements and experiments.
The Daniell Cell is an example of a voltaic cell.
As a voltaic cell, the Daniell Cell converts chemical energy into electrical energy through a spontaneous redox reaction. This principle continues to be fundamental in battery technology.
Conclusion
In conclusion, the Daniell cell is a remarkable invention that revolutionized the field of electrochemistry. From its inception by John Frederic Daniell in the early 19th century, this electrochemical cell has been widely used and studied. Its ability to generate a stable and constant electric current made it an essential component in various applications, including telegraph systems and early battery technology.
The Daniell cell’s design and principles are still relevant today, with modern advancements in electrochemical technology building upon its foundation. Its importance in understanding the concept of electrochemical cells and the role of redox reactions cannot be overstated. Through the Daniell cell, scientists and researchers have gained valuable insights into the complexities of chemical reactions and the generation of electrical energy.
As we continue to explore the fascinating world of chemistry, it is crucial to recognize and appreciate the contributions of the Daniell cell in shaping our understanding of electrochemistry and its practical applications.
FAQs
Q: What is a Daniell cell?
A: The Daniell cell is an early type of electrochemical cell that was invented by John Frederic Daniell in the early 19th century. It consists of a copper electrode immersed in a copper sulfate solution, a zinc electrode immersed in a zinc sulfate solution, and a porous barrier separating the two solutions.
Q: How does a Daniell cell work?
A: The Daniell cell works through a redox reaction where copper ions are reduced at the copper electrode, and zinc metal is oxidized at the zinc electrode. This creates a flow of electrons, generating an electric current that can be harnessed for various applications.
Q: What are the applications of a Daniell cell?
A: The Daniell cell has been used in early telegraph systems, where it provided a stable and constant source of electric current. It also played a significant role in the development of battery technology and as a fundamental tool for studying the principles of electrochemistry.
Q: Is the Daniell cell still used today?
A: While more advanced and efficient cell designs have been developed since the invention of the Daniell cell, its principles and concepts are still relevant in modern electrochemical applications. It serves as a foundational model for understanding electrochemical cells and their practical uses.
Q: What are the advantages of a Daniell cell?
A: The Daniell cell offers a stable and constant source of electrical energy, making it suitable for applications that require long-lasting power. It also provided insights into the field of electrochemistry, leading to further advancements in battery technology and electrical systems.
The Daniell cell's fascinating history and impact on electrochemistry make it a captivating subject for those curious about scientific discoveries. Exploring its connection to other groundbreaking inventions, such as the galvanic cell, can further deepen one's appreciation for the field of electrochemistry. Moreover, learning about the Daniell cell's role in the development of modern technologies, like fuel cells that convert chemical energy into electricity, showcases the ongoing importance of these fundamental concepts.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
