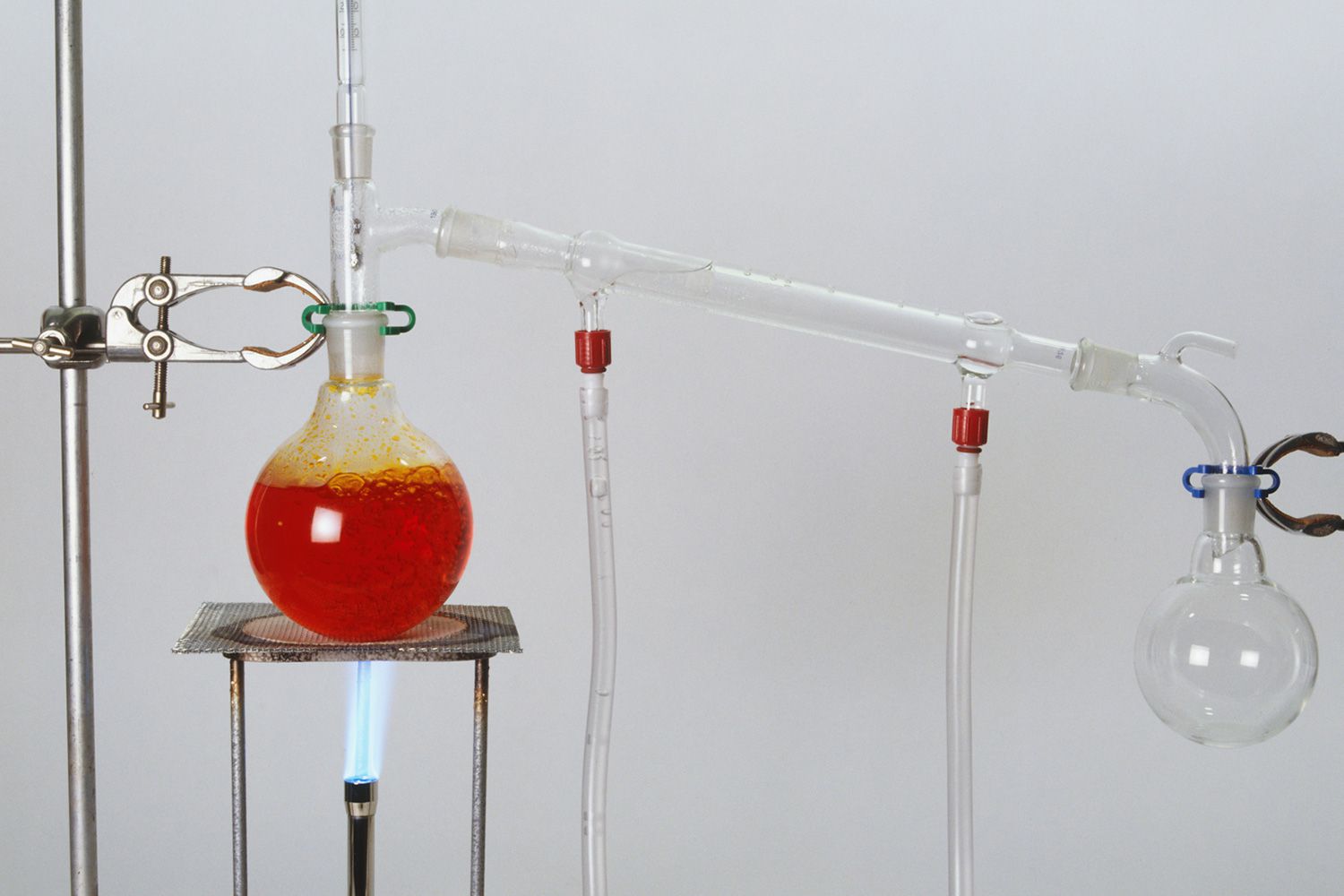
Distillation is a fascinating process that has been utilized for centuries to separate mixtures and extract essential substances. It holds great significance in the field of chemistry, finding applications in various industries such as pharmaceuticals, oil refining, alcohol production, and so much more. In essence, distillation involves heating a mixture to convert the components into vapor, and then cooling and condensing the vapor to obtain the desired product. It is a highly effective method for purifying liquids, isolating volatile compounds, and even separating complex chemical mixtures. In this article, we will delve into the intriguing world of distillation and explore 13 captivating facts that will not only expand your knowledge but also deepen your appreciation for this essential process.
Key Takeaways:
- Distillation is a cool process that separates mixtures based on their boiling points. It’s used to make drinks, separate oil, and even extract essential oils from plants!
- Did you know distillation has been around for thousands of years? It’s super important in chemistry, helps make medicines, and even recycles solvents to reduce waste!
Distillation is a process of separating mixtures.
Distillation is a method used to separate the components of a mixture based on their different boiling points. It is widely used in various industries, including chemistry, petroleum, and beverage production.
The origin of distillation dates back thousands of years.
The process of distillation has been practiced for centuries. It was first documented in ancient Greece and was further developed by Islamic scientists during the Middle Ages.
Distillation played a crucial role in the development of modern chemistry.
Distillation techniques paved the way for advancements in the field of chemistry, allowing scientists to separate and isolate various chemical compounds for further study and understanding.
There are different types of distillation methods.
Some commonly used distillation methods include simple distillation, fractional distillation, and steam distillation. Each method has its own unique applications and advantages.
Distillation is used in the production of alcoholic beverages.
The distillation process is integral to the production of spirits such as whiskey, vodka, and rum. It helps to purify the liquid, remove impurities, and increase the alcohol content.
Distillation is also utilized in the petroleum industry.
In the petroleum industry, distillation is used to separate crude oil into different components like gasoline, diesel, and jet fuel. This process is known as fractional distillation.
Distilled water is not just water.
Distilled water is produced through the process of distillation, where impurities and minerals are removed. It is commonly used in laboratories, medical procedures, and even in steam irons.
Distillation is used to extract essential oils from plants.
In the field of aromatherapy and perfumery, distillation is employed to extract the aromatic oils from various plants and flowers. This method helps in preserving the scent and therapeutic properties of the oils.
Distillation can purify and concentrate substances.
The separation capabilities of distillation allow for the purification and concentration of substances. This is particularly useful in the pharmaceutical industry for the production of drugs and medications.
Distillation can be used to recycle and reclaim solvents.
In industrial processes, distillation can be utilized to recover and recycle solvents, reducing waste and minimizing environmental impact.
Distillation is not limited to liquids.
While distillation is commonly associated with liquid mixtures, it can also be applied to separate and purify gases, such as in the production of liquefied petroleum gas (LPG).
Distillation requires a heat source.
In order to separate the components of a mixture through distillation, a heat source is necessary to vaporize the substances with lower boiling points.
Distillation is an essential process in the production of ethanol for fuel.
The distillation of fermented solutions, such as ethanol, is crucial in the production of biofuels, providing an alternative and sustainable energy source.
These 13 fascinating facts about distillation highlight the importance and versatility of this process in various industries. Whether it’s separating a mixture, purifying substances, or producing alcoholic beverages and essential oils, distillation plays a crucial role in our everyday lives.
Conclusion
In conclusion, distillation is a fascinating process that plays a vital role in various industries and scientific fields. It is a method used to separate and purify different substances based on their boiling points. Through the process of heating and condensation, volatile substances are vaporized and then collected as a liquid.Distillation has numerous practical applications, from producing alcoholic beverages and essential oils to refining petroleum and creating pharmaceutical drugs. It is a fundamental technique in chemical research and analysis, enabling scientists to obtain pure compounds and study their properties.Understanding the process of distillation helps us appreciate the complexity and importance of this technique. Whether you’re a chemistry enthusiast or simply curious about the world around you, exploring the world of distillation will undoubtedly expand your knowledge and appreciation for the wonders of science.
FAQs
Q: What is distillation?
A: Distillation is a process used to separate mixtures by exploiting differences in boiling points. It involves heating a mixture to vaporize the volatile substances and then condensing the vapor to collect the purified liquid phase.
Q: What are the common applications of distillation?
A: Distillation is widely used in various industries, including the production of alcoholic beverages, essential oils, petroleum refining, and pharmaceutical manufacturing. It is also used in laboratories for research and analysis purposes.
Q: How does distillation work?
A: Distillation works by heating a mixture to its boiling point to convert the volatile components into vapor. The vapor is then cooled and condensed back into a liquid, where it can be collected separately from the other components of the mixture.
Q: Can distillation be used to purify all substances?
A: Distillation is an effective method for separating substances with different boiling points. However, it may not be suitable for mixtures where the components have similar boiling points or undergo chemical reactions during the process.
Q: Are there different types of distillation?
A: Yes, there are different types of distillation, including simple distillation, fractional distillation, and vacuum distillation. Each method is suitable for specific applications and allows for more precise separation of substances.
Q: Is distillation an energy-intensive process?
A: Yes, distillation can require a significant amount of energy, especially when separating substances with close boiling points. However, advancements in technology have led to the development of more energy-efficient distillation methods.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
