Chemistry is a fascinating subject that deals with the composition, properties, and transformations of matter. Within this vast field, there are numerous concepts and principles that students and scientists delve into to gain a deeper understanding of the world around us.
One such concept is the measurement of acidity and basicity, which plays a crucial role in various chemical reactions and phenomena. Kw and pKw are terms that are commonly encountered in discussions related to acid-base chemistry. They are used to quantify the strength of acids and bases and provide valuable insights into their behavior.
In this article, we will explore 11 intriguing facts about Kw and pKw, shedding light on their significance and how they contribute to our understanding of the acidic and basic nature of substances. So, let’s dive into the world of chemistry and unravel the mysteries behind these fascinating concepts.
Key Takeaways:
- Kw is the magic number for water! It shows how water breaks apart into ions, and its value remains constant, helping scientists understand the strength of acids and bases.
- pH and pOH are like secret codes for acidity and basicity. They help scientists unlock the mysteries of solutions and predict their behavior.
What is Kw?
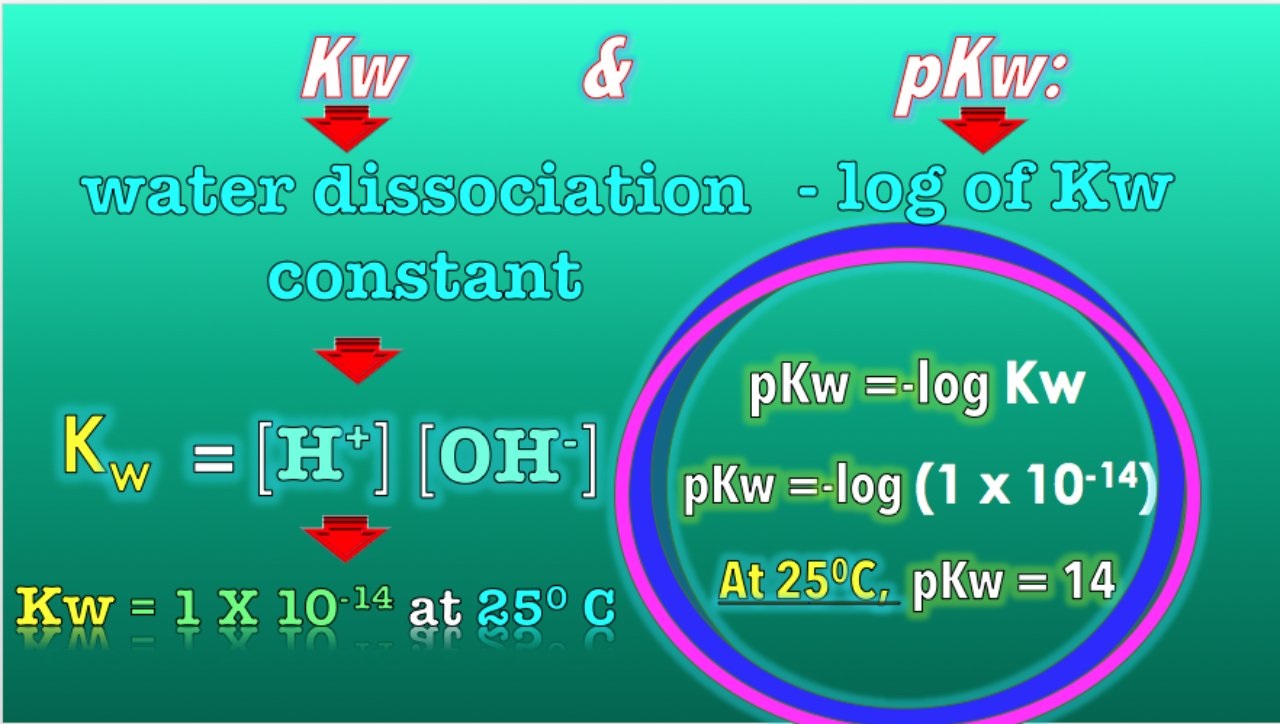
Fact: Kw represents the ionization constant of water.
Water is not a static compound; it can dissociate into ions known as hydronium (H3O+) and hydroxide (OH-) ions through a process called autoionization. Kw is the equilibrium constant for this reaction and has a fixed value at a given temperature.
The Value of Kw
Fact: Kw has a value of 1.0 x 10^-14 at 25 degrees Celsius.
This constant reflects the extent of the autoionization of water. The concentration of H3O+ ions multiplied by the concentration of OH- ions in pure water is always equal to Kw.
pH and pOH
Fact: pH and pOH are logarithmic measurements of hydrogen ion concentration and hydroxide ion concentration, respectively.
pH is calculated by taking the negative logarithm of the H3O+ ion concentration, while pOH is calculated using the OH- ion concentration. These values are essential in determining the acidity or basicity of a solution.
pKw
Fact: pKw is the negative logarithm of Kw.
It is a useful measure to indicate the acidity or basicity of a solution. An acidic solution will have a pKw value less than 14, while a basic solution will have a pKw value greater than 14.
Relationship between pH and pOH
Fact: pH + pOH = 14
This mathematical relationship holds true for any aqueous solution. If you know the value of pH or pOH, you can easily calculate the other one using this equation.
Kw and Temperature
Fact: Kw increases with an increase in temperature.
As the temperature rises, the autoionization of water becomes more favorable, resulting in a higher concentration of H3O+ and OH- ions. Consequently, the value of Kw increases.
Kw in Acidic and Basic Solutions
Fact: Kw remains constant in acidic and basic solutions.
Although the concentration of H3O+ and OH- ions differs in acidic and basic solutions, their product (Kw) remains the same. This fact is a fundamental principle of chemistry.
Neutral Solutions
Fact: In neutral solutions, the concentration of H3O+ ions is equal to the concentration of OH- ions.
In a neutral solution, the pH is 7, and both H3O+ and OH- ions have equal concentrations. This balance is essential for the neutrality of the solution.
Kw and the Kw Concentration Constant
Fact: The concentration constant for Kw is always equal to the square of the H3O+ or OH- ion concentration in a neutral solution.
This concept is vital in determining the ion concentration in solutions and understanding their behavior.
Kw and Acid-Base Reactions
Fact: Kw is closely related to acid-base reactions.
Understanding the value of Kw helps in predicting and analyzing the behavior of acids and bases in different solutions. It provides valuable insights into chemical reactions and their equilibrium.
Importance of Kw in Analytical Chemistry
Fact: Kw is crucial in various analytical techniques and experiments.
By considering the value of Kw, scientists can determine other properties of solutions, such as the concentration of unknown substances or the strength of acids and bases. It serves as a fundamental tool in the field of analytical chemistry.
These 11 intriguing facts about Kw and pKw shed light on the remarkable properties of water, its ionization, and its significance in acid-base chemistry. By gaining knowledge about Kw and pKw, scientists can better understand the behavior of substances in aqueous solutions and make significant contributions to various fields of research.
Conclusion
In conclusion, understanding the concepts of Kw and pKw is vital in the field of chemistry. These terms play a crucial role in determining the acidity or basicity of a solution and are used extensively in various calculations. Kw represents the equilibrium constant for the autoionization of water, while pKw is the negative logarithm of Kw. These values are fundamental in understanding the pH scale and its applications.
By exploring the 11 intriguing facts about Kw and pKw, we have uncovered the significance and complexity of these concepts. From their relationship to temperature to their role in determining the strength of acids and bases, these facts provide valuable insights into the world of aqueous chemistry.
Whether you are a chemistry student, a professional in the field, or simply someone curious about the world around us, familiarizing yourself with Kw and pKw is essential for a comprehensive understanding of chemical interactions and their implications.
FAQs
1. What does Kw stand for?
Kw stands for the equilibrium constant for the autoionization of water.
2. How is pKw calculated?
pKw is calculated by taking the negative logarithm of Kw.
3. What is the significance of Kw in chemistry?
Kw is significant as it determines the concentration of H+ and OH- ions in a solution, thereby influencing its acidity or basicity.
4. Is Kw affected by temperature?
Yes, Kw is affected by temperature. As temperature increases, the value of Kw also increases.
5. What is the relationship between Kw and pH?
Kw is related to the pH scale as it determines the concentration of H+ ions, which directly influences the pH of a solution.
6. Can Kw be used to calculate the concentration of H+ ions?
Yes, by knowing the value of Kw and the concentration of either H+ or OH- ions, we can calculate the concentration of the other ion.
7. How is pKw related to acidity and basicity?
The value of pKw can be used to determine the acidity or basicity of a solution. A low pKw value indicates an acidic solution, while a high pKw value indicates a basic solution.
8. Can Kw be used to compare the strengths of different acids and bases?
Yes, the value of Kw can be used to compare the strengths of different acids and bases. A higher Kw value indicates a stronger acid-base behavior.
9. Are there any exceptions to the calculation of pKw?
In very concentrated solutions or non-aqueous solutions, the calculation of pKw may not be applicable.
10. How does temperature affect the value of pKw?
As temperature increases, the value of pKw decreases. This means that the solution becomes more acidic.
11. Can pKw be used to predict the behavior of a solution?
Yes, by knowing the pKw value, we can predict the behavior of a solution. A lower pKw value indicates a higher concentration of H+ ions, making the solution more acidic, while a higher pKw value indicates a higher concentration of OH- ions, making the solution more basic.
Exploring the intriguing world of Kw and pKw is just the beginning of your chemistry journey. Dive deeper into chemical equilibrium by learning about the equilibrium constant (Kc) and its fascinating properties. Don't forget to brush up on your understanding of acidity and alkalinity with mind-blowing facts about the pH scale. Embark on a quest to unravel the mysteries of chemistry and expand your knowledge of these fundamental concepts.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
