Enzyme kinetics, a fascinating field of study within biology, delves into the intricate mechanisms behind the speed and efficiency of enzymatic reactions. Enzymes, often referred to as the “biological catalysts,” play a crucial role in countless physiological processes that sustain life. Understanding the kinetics of enzymes provides insights into how these catalysts work, their efficiency, and their regulation within biological systems.
In this article, we will explore 19 surprising facts about enzyme kinetics that will deepen your understanding of this captivating subject. From the diverse groups of enzymes and their classifications to the factors influencing enzymatic activity, we will dive into the intricate details that govern the fascinating world of enzyme kinetics. Let’s embark on this journey to uncover some unexpected facts and broaden our understanding of the remarkable functions of enzymes in biological systems.
Key Takeaways:
- Enzyme kinetics studies the speed of enzymes in living organisms and is influenced by factors like pH and temperature. It’s like understanding how fast and under what conditions enzymes work in our bodies.
- Enzyme-substrate binding is reversible, and enzymes can exhibit different catalytic mechanisms. It’s like a lock and key that can open and close multiple times, and enzymes have different ways of making reactions happen.
Enzymes are biological catalysts.
Enzymes play a critical role in speeding up chemical reactions in living organisms, without undergoing any permanent changes themselves.
Enzyme kinetics studies the rate of enzyme-catalyzed reactions.
Enzyme kinetics is the branch of biochemistry that focuses on understanding the rates at which enzymes catalyze biochemical reactions.
Enzyme kinetics is influenced by factors such as pH and temperature.
The activity of enzymes is highly dependent on the optimal pH and temperature range for their function.
Enzyme-substrate binding is reversible.
The binding of an enzyme to its substrate is not a one-time event; it can occur and dissociate multiple times.
Enzymes can exhibit different catalytic mechanisms.
Enzymes can catalyze reactions through various mechanisms, including acid-base catalysis, covalent catalysis, and metal ion catalysis.
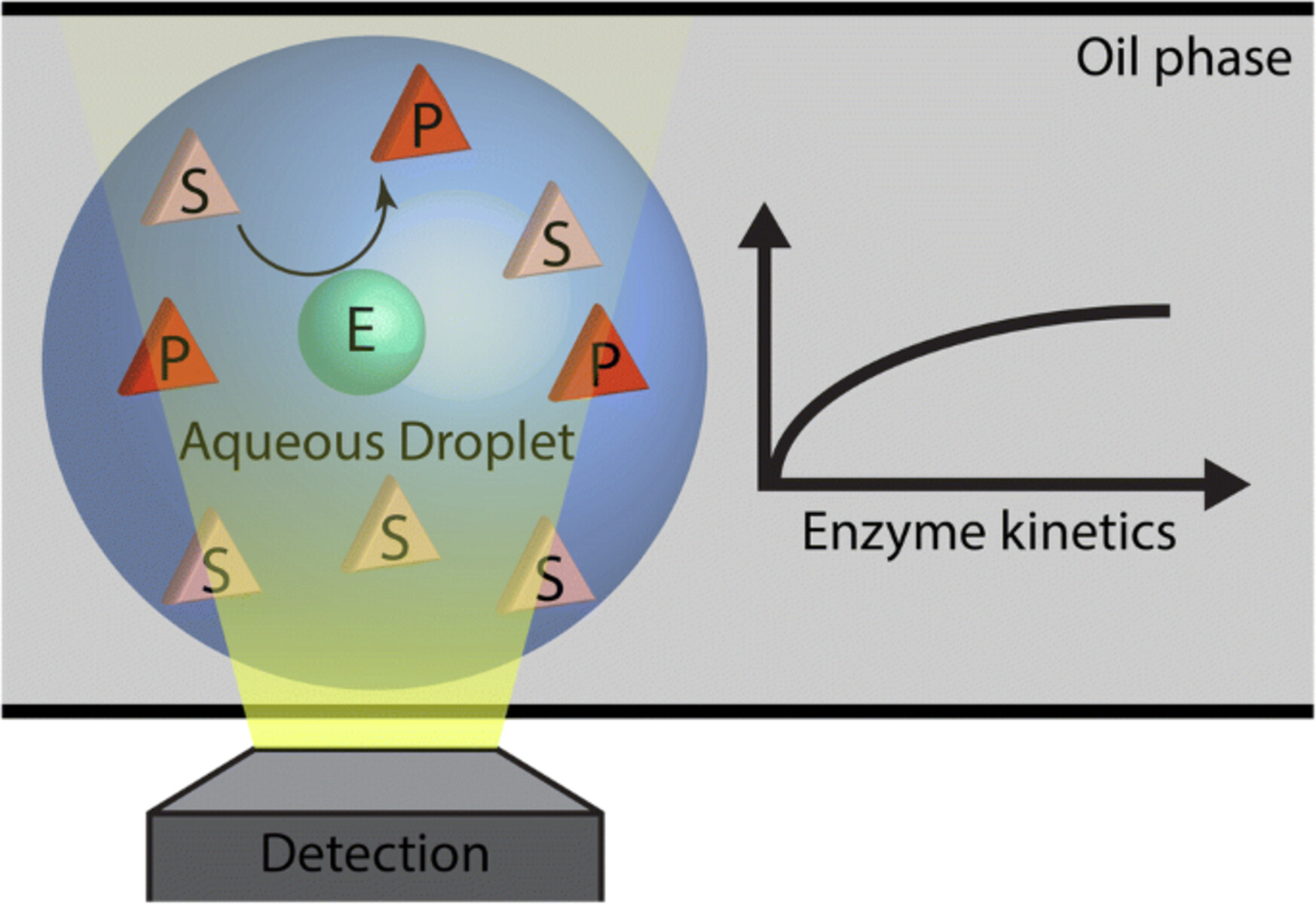
Enzyme kinetics can be described by the Michaelis-Menten equation.
The Michaelis-Menten equation is a mathematical model that describes the rate of enzyme-catalyzed reactions based on substrate concentration.
Enzymes can undergo allosteric regulation.
Allosteric regulation occurs when a molecule binds to a site on the enzyme, causing a change in its catalytic activity.
Enzyme inhibitors can block the active site.
Competitive and non-competitive inhibitors can bind to the active site of an enzyme, preventing substrate binding and catalysis.
Enzyme kinetics can be measured using spectrophotometry.
Spectrophotometry is a common technique used to measure the absorbance or emission of light by enzyme-substrate complexes.
Enzyme activity can be affected by enzyme concentration.
The rate of an enzyme-catalyzed reaction can increase or decrease with changes in enzyme concentration, as long as there is excess substrate available.
Enzymes can exhibit cooperativity.
In enzymes with multiple subunits, substrate binding to one subunit can influence the activity of the other subunits.
Enzymes can be denatured.
Extreme pH and temperature conditions can disrupt the structure of enzymes, rendering them inactive.
Enzyme kinetics can be affected by enzyme-substrate ratios.
The ratio of enzyme to substrate can impact the rate of the reaction, with optimal ratios leading to maximum catalytic efficiency.
Enzyme kinetics can follow different reaction orders.
Enzyme-catalyzed reactions can exhibit zero-order, first-order, or second-order kinetics, depending on the rate-limiting steps involved.
Enzyme kinetics can be influenced by the presence of activators.
Activators can enhance the catalytic activity of enzymes by increasing their affinity for substrate or inducing conformational changes.
Enzymes can exhibit pH-dependent activity profiles.
The optimal pH for enzyme activity can vary among different enzymes, reflecting their adaptation to specific biological environments.
Enzymes can display substrate specificity.
Each enzyme has a particular substrate or group of substrates that it can bind and catalyze reactions with, ensuring specificity in biochemical pathways.
Enzyme kinetics can be affected by enzyme cofactors.
Cofactors, such as vitamins or metal ions, can interact with enzymes and assist in catalysis by providing additional chemical groups or modifying the enzyme’s structure.
Enzyme kinetics is crucial for understanding drug metabolism.
By studying enzyme kinetics, scientists can gain insights into how drugs are metabolized in the body, leading to better drug design and dosing strategies.
Understanding the processes of enzyme kinetics is essential for comprehending the intricate workings of biochemical reactions in living systems. The 19 surprising facts about enzyme kinetics highlight the diverse and fascinating aspects of this field. From understanding enzyme-substrate interactions to exploring the factors influencing enzyme activity, enzyme kinetics plays a central role in understanding the intricate mechanisms of life itself.
Conclusion
In conclusion, enzyme kinetics is a fascinating field of study that plays a crucial role in understanding the mechanisms behind enzymatic reactions. Through the various surprising facts discussed in this article, we have seen how enzymes undergo complex processes to catalyze reactions, how their activity can be altered by environmental factors, and how enzyme kinetics can be quantitatively analyzed.
Understanding enzyme kinetics is not only important in the field of biology but also has significant implications in various applications, including medicine, biochemistry, and biotechnology. By studying and manipulating enzyme kinetics, researchers can develop new pharmaceuticals, improve industrial processes, and gain insights into metabolic disorders.
Overall, enzyme kinetics provides us with a deeper understanding of the intricate machinery that drives biological reactions. By unraveling the mysteries of enzyme kinetics, scientists continue to make innovative discoveries that have the potential to revolutionize the world we live in.
FAQs
1. What is enzyme kinetics?
Enzyme kinetics is the study of the rates at which enzymes catalyze reactions and the factors that affect their activity
2. Why is enzyme kinetics important?
Enzyme kinetics helps us understand how enzymes work, which is crucial for fields such as medicine, biochemistry, and biotechnology.
3. What factors can affect enzyme activity?
Enzyme activity can be influenced by factors such as temperature, pH, substrate concentration, and the presence of inhibitors or activators.
4. How is enzyme activity quantitatively analyzed?
Enzyme activity can be measured using parameters such as the initial reaction rate, Michaelis-Menten kinetics, and the Lineweaver-Burk plot.
5. Are all enzymes the same in terms of kinetics?
No, enzymes can have different kinetic properties depending on their structure, function, and specific reaction they catalyze.
6. Can enzyme kinetics be applied in real-world applications?
Absolutely! Enzyme kinetics is widely applied in various industries, including pharmaceuticals, food processing, and environmental monitoring.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.