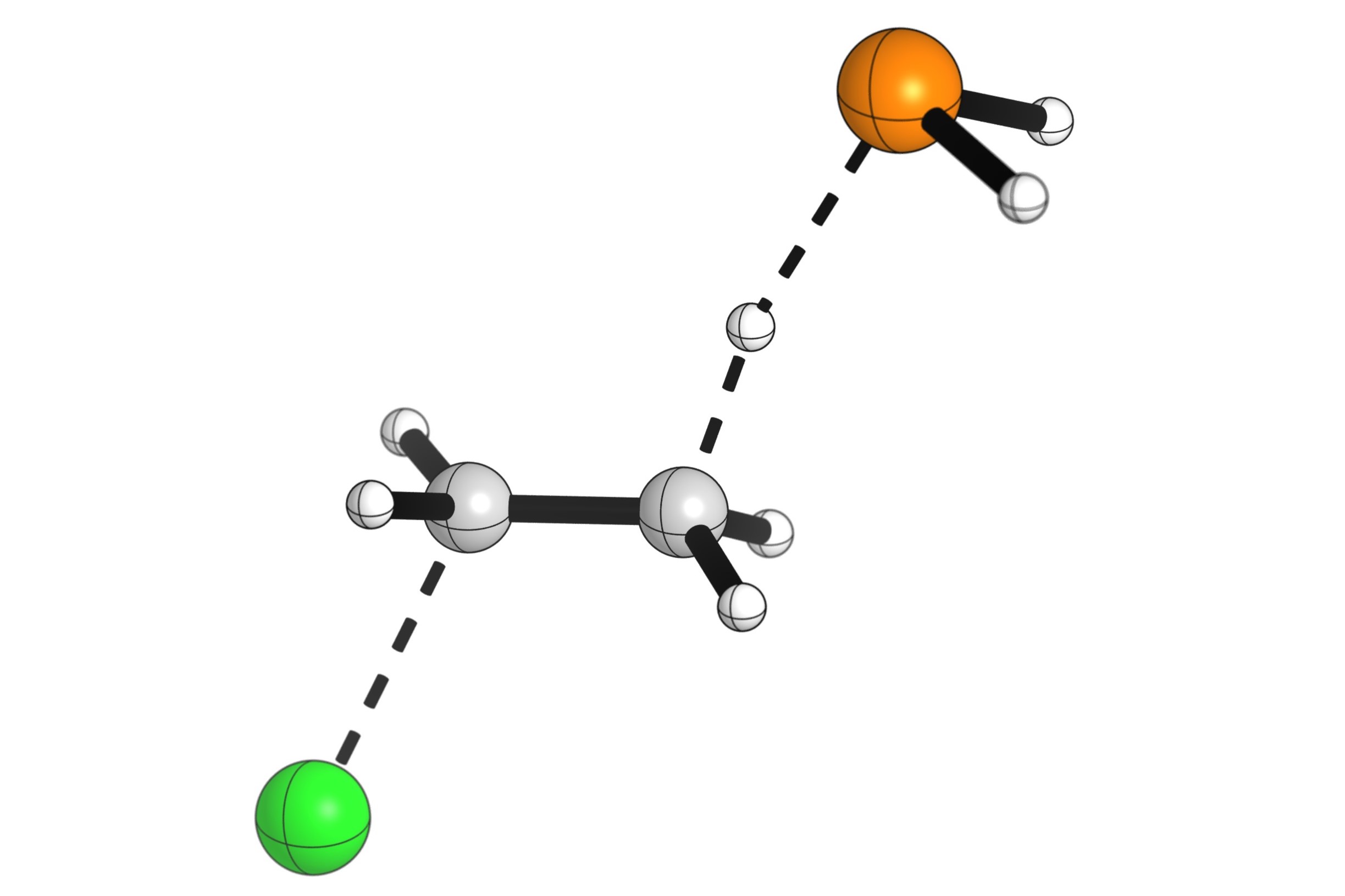
What is an elimination reaction? In chemistry, an elimination reaction involves the removal of two atoms or groups from a molecule, forming a new double bond or ring structure. These reactions are crucial in organic chemistry, helping create alkenes and alkynes. They typically occur in the presence of a base or acid, which facilitates the removal process. There are two main types: E1 and E2. E1 reactions happen in two steps, while E2 reactions occur in one concerted step. Understanding these reactions can help in synthesizing complex molecules and designing new drugs. Ready to dive into the world of elimination reactions? Let's get started!
What is an Elimination Reaction?
An elimination reaction is a type of organic reaction where two substituents are removed from a molecule, resulting in the formation of a double bond or a ring structure. These reactions are crucial in organic chemistry, especially in the synthesis of alkenes and alkynes.
-
Elimination reactions often involve the removal of a hydrogen atom and a leaving group, such as a halide or a hydroxyl group.
-
There are two main types of elimination reactions: E1 (unimolecular elimination) and E2 (bimolecular elimination).
-
E1 reactions proceed through a two-step mechanism involving the formation of a carbocation intermediate.
-
E2 reactions occur in a single step, where the base removes a proton while the leaving group departs simultaneously.
E1 vs. E2 Reactions
Understanding the differences between E1 and E2 reactions is essential for mastering organic chemistry. Each type has unique characteristics and conditions under which they occur.
-
E1 reactions are typically favored in polar protic solvents, which stabilize the carbocation intermediate.
-
E2 reactions are favored in polar aprotic solvents, which do not stabilize carbocations but enhance the nucleophilicity of the base.
-
E1 reactions often occur with tertiary alkyl halides due to the stability of the resulting carbocation.
-
E2 reactions can occur with primary, secondary, or tertiary alkyl halides, depending on the strength of the base.
Factors Affecting Elimination Reactions
Several factors influence whether an elimination reaction will proceed via the E1 or E2 mechanism. These include the nature of the substrate, the strength of the base, and the solvent used.
-
Steric hindrance plays a significant role in determining the pathway of the reaction. Bulky bases favor E2 mechanisms.
-
Temperature can also affect the reaction. Higher temperatures generally favor elimination over substitution.
-
Leaving group ability is crucial. Better leaving groups facilitate both E1 and E2 reactions.
-
Base strength is more critical in E2 reactions, where a strong base is required to remove the proton.
Applications of Elimination Reactions
Elimination reactions are not just academic exercises; they have practical applications in various fields, including pharmaceuticals, petrochemicals, and materials science.
-
Alkene synthesis is one of the primary applications of elimination reactions, providing a route to create double bonds in organic molecules.
-
Dehydration of alcohols is a common elimination reaction used to produce alkenes.
-
Dehydrohalogenation involves the elimination of a hydrogen halide from an alkyl halide to form an alkene.
-
Polymerization processes often rely on elimination reactions to create monomers with double bonds.
Real-World Examples
Elimination reactions are not just confined to the lab. They occur in various real-world processes and industrial applications.
-
Cracking of hydrocarbons in the petrochemical industry involves elimination reactions to break down large molecules into smaller, more useful ones.
-
Synthesis of pharmaceuticals often involves elimination steps to create the desired molecular structures.
-
Biological systems also utilize elimination reactions, such as the formation of double bonds in fatty acid metabolism.
-
Environmental chemistry uses elimination reactions to degrade pollutants and create less harmful substances.
Mechanistic Insights
Delving into the mechanisms of elimination reactions can provide deeper insights into how these processes occur at the molecular level.
-
Transition states in E2 reactions involve a concerted mechanism where bond-breaking and bond-forming occur simultaneously.
-
Carbocation stability is a key factor in E1 reactions, with more stable carbocations leading to faster reactions.
-
Anti-periplanar geometry is often required in E2 reactions, where the proton and leaving group must be aligned opposite each other.
-
Zaitsev's rule predicts that the more substituted alkene will be the major product in elimination reactions.
Common Misconceptions
There are several misconceptions about elimination reactions that can lead to confusion. Clarifying these can help in better understanding the topic.
-
E1 and E2 reactions are not always mutually exclusive; some reactions can proceed via both mechanisms under different conditions.
-
Substitution and elimination can compete, especially in reactions involving alkyl halides.
-
Carbocation rearrangements can occur in E1 reactions, leading to unexpected products.
-
Base strength does not always correlate with nucleophilicity; a strong base may not be a good nucleophile.
Advanced Topics
For those looking to delve deeper, there are advanced topics within elimination reactions that offer further complexity and intrigue.
-
E1cB mechanism involves a carbanion intermediate and is a variant of the E1 mechanism.
-
Stereochemistry plays a crucial role in elimination reactions, affecting the configuration of the resulting alkenes.
-
Regioselectivity in elimination reactions can be influenced by the structure of the substrate and the conditions used.
-
Kinetic isotope effects can provide insights into the mechanism of elimination reactions by comparing the rates of reactions involving isotopically labeled substrates.
Experimental Techniques
Various experimental techniques can be used to study and optimize elimination reactions, providing valuable data for chemists.
-
NMR spectroscopy can be used to monitor the progress of elimination reactions and identify intermediates.
-
Mass spectrometry helps in determining the molecular weight and structure of the products formed.
-
Infrared spectroscopy can identify functional groups and monitor changes during the reaction.
-
Chromatography is essential for separating and purifying the products of elimination reactions.
Final Thoughts on Elimination Reactions
Elimination reactions play a crucial role in organic chemistry. They help form double and triple bonds, which are essential for creating complex molecules. Understanding the mechanisms, such as E1 and E2, can provide insights into reaction conditions and outcomes. These reactions are not just academic; they have practical applications in pharmaceuticals, petrochemicals, and materials science. Knowing the factors that influence elimination reactions, like the nature of the leaving group and the strength of the base, can help predict and control chemical processes. Whether you're a student, a researcher, or just curious, grasping these concepts can open doors to deeper chemical knowledge. Keep exploring, experimenting, and asking questions. Chemistry is a vast field with endless possibilities.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


