The isothermal process is a fascinating concept in physics that involves a unique set of conditions and properties. This process refers to a scenario where the temperature of a system remains constant throughout the entire process, while other parameters such as pressure and volume may change. Isothermal processes have significant applications in various fields, including thermodynamics, gas dynamics, and engineering. In this article, we will delve into the world of isothermal processes and discover 12 astonishing facts that shed light on their intriguing nature. From the laws governing these processes to their practical implications, we will explore the captivating aspects of isothermal processes that make them an essential area of study in the realm of physics. So, let’s embark on a journey of discovery and unravel the mysteries behind isothermal processes.
Key Takeaways:
- Isothermal processes keep things cool! They maintain a constant temperature, like when your ice cream stays frozen while you eat it. This helps in understanding gases and energy conservation.
- Isothermal processes are like magic tricks for science! They help in precise measurements and understanding how things change without getting too hot or too cold. It’s like a special superpower for scientists!
The Isothermal Process Maintains Constant Temperature
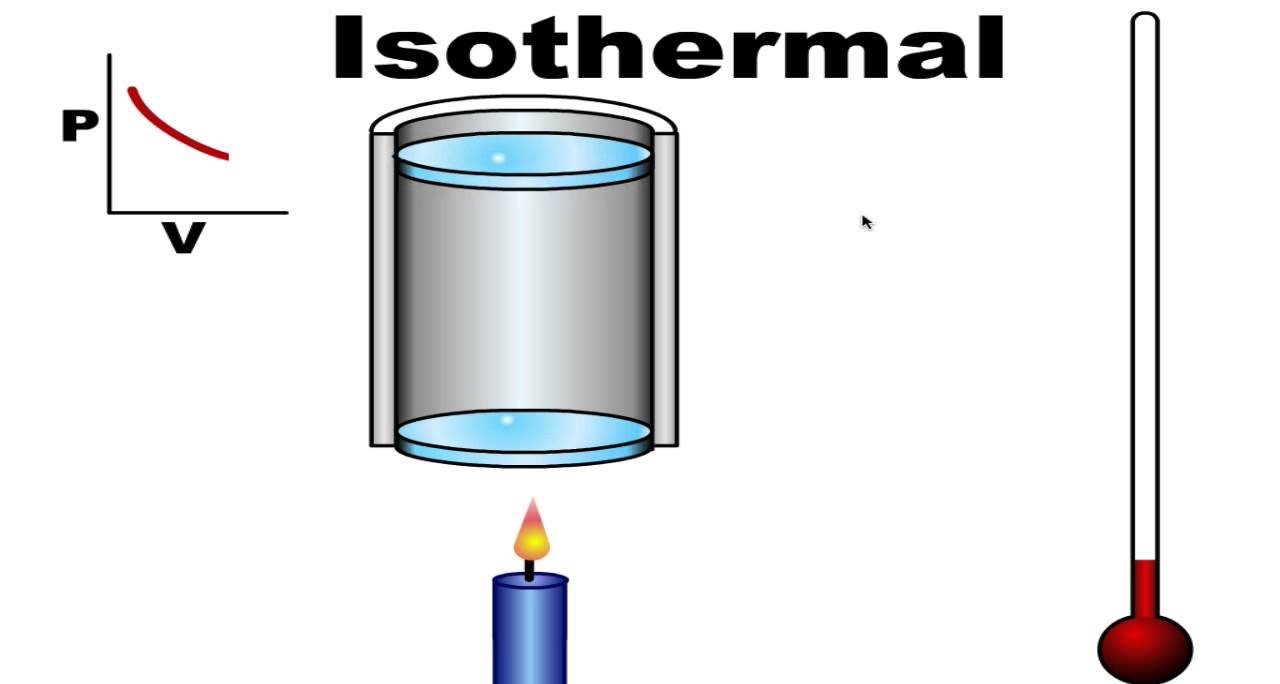
The isothermal process is a thermodynamic process in which the temperature of a system remains constant. This means that as the system undergoes a change, such as expansion or compression, the temperature stays the same throughout the process.
Isothermal Expansion and Compression Occur in Real Life
Isothermal expansion and compression are not just theoretical concepts, but they also occur in real-life scenarios. For example, the expansion and compression of gases in engines, such as in the cylinders of an internal combustion engine, can be approximated as isothermal processes.
Isothermal Process Is Represented by a Horizontal Line on a Pressure-Volume Graph
On a pressure-volume graph, an isothermal process is represented by a horizontal line. This is because, during an isothermal process, the product of pressure and volume remains constant.
The Work Done on or by the System in an Isothermal Process Depends on the Gas
The work done on or by the system during an isothermal process is dependent on the type of gas involved. For an ideal gas, the work done is given by the equation W = nRT ln(Vf/Vi), where W is the work done, n is the number of moles of gas, R is the ideal gas constant, T is the temperature, Vi is the initial volume, and Vf is the final volume.
Isothermal Process Can Be Achieved by Slow Heat Transfer
To achieve an isothermal process, heat transfer must occur slowly. This allows the system to continuously exchange heat with its surroundings to maintain a constant temperature. This slow heat transfer helps minimize any temperature gradients within the system.
The Isothermal Process is Reversible
An isothermal process is considered reversible because it can be reversed without any change in the system or its surroundings. This means that if the process is carried out in the reverse direction, the system will return to its initial state.
Isothermal Process can be Used in Heat Engines
The isothermal process plays a crucial role in the operation of heat engines, such as the Stirling engine. In these engines, the working fluid undergoes isothermal expansion and compression, allowing for the efficient conversion of thermal energy into mechanical work.
Isothermal Process Helps in Understanding Ideal Gas Behavior
The study of isothermal processes is fundamental in understanding the behavior of ideal gases. It helps explain the relationship between pressure, volume, and temperature for an ideal gas, as described by the ideal gas law: PV = nRT.
The Isothermal Process ensures Energy Conservation
During an isothermal process, energy is conserved since the temperature remains constant. This conservation of energy is a fundamental principle in thermodynamics and is crucial in various engineering applications.
The Isothermal Process Relates to Reversible Heat Transfer
The isothermal process is closely related to reversible heat transfer, where heat flows between two bodies at a constant temperature. This concept is often utilized in various cooling and heating applications, such as in refrigerators and air conditioners.
Isothermal Process Helps in Understanding Phase Transitions
The study of isothermal processes is not limited to ideal gases but also extends to the understanding of phase transitions. For example, the process of water boiling at a constant temperature is an isothermal process, where the added energy is utilized to break intermolecular bonds rather than increasing the temperature.
The Isothermal Process Enables Precise Measurements
The isothermal process provides a means for precise measurements in various scientific experiments. By maintaining a constant temperature, scientists can accurately measure and analyze the changes occurring in a system without the confounding factor of temperature fluctuations.
Conclusion
In conclusion, isothermal processes are truly fascinating and have a significant impact in the field of physics. Their unique characteristics and properties make them crucial in various applications, from thermodynamics to modern engineering. Understanding isothermal processes allows us to comprehend the behavior of gases and their transformations in a controlled environment. From the gas laws to the practical applications in heat engines and refrigeration systems, isothermal processes play a crucial role in our everyday lives.
FAQs
1. What is an isothermal process?
An isothermal process is a thermodynamic process that occurs at a constant temperature. In this process, the system exchanges heat with its surroundings to maintain a constant temperature throughout.
2. What are the characteristics of an isothermal process?
An isothermal process is characterized by constant temperature, slow heat transfer, and a smooth, horizontal line on a pressure-versus-volume graph.
3. What is the importance of isothermal processes?
Isothermal processes are important in various fields, such as thermodynamics, engineering, and chemistry. They provide insights into the behavior of gases and help in the design of efficient heat engines and refrigeration systems.
4. How does an isothermal process differ from an adiabatic process?
In an isothermal process, the temperature remains constant, while in an adiabatic process, no heat is exchanged with the surroundings. Adiabatic processes generally involve rapid changes in temperature.
5. Can you give an example of an isothermal process?
An example of an isothermal process is the expansion or compression of a gas in a piston-cylinder system, where the system is in thermal equilibrium with its surroundings, and the temperature remains constant.
6. Are isothermal processes reversible?
Isothermal processes can be reversible if the system is in thermodynamic equilibrium throughout the process and no irreversible factors like friction come into play. However, in real-world scenarios, achieving complete reversibility is often challenging.
7. How are isothermal processes used in refrigeration?
Isothermal processes are used in refrigeration systems to achieve cooling. By removing heat from the refrigerated space and maintaining a constant temperature during the cooling process, isothermal processes ensure efficient and effective refrigeration.
8. How do isothermal processes relate to the ideal gas law?
Isothermal processes obey the ideal gas law, which states that the pressure of a gas is inversely proportional to its volume when temperature and the number of gas molecules remain constant.
9. Can an isothermal process occur in a closed system?
Yes, an isothermal process can occur in a closed system. In a closed system, heat is exchanged with the surroundings to maintain a constant temperature, resulting in an isothermal process.
10. Are all processes in nature isothermal?
No, not all processes in nature are isothermal. Most natural processes involve temperature changes, and isothermal processes are a special case that occurs under specific conditions of heat transfer and thermal equilibrium.
Isothermal processes captivate scientists and engineers alike, but thermodynamics holds even more fascinating concepts. Delve into the intricacies of the Carnot cycle, a theoretical heat engine that achieves maximum efficiency. This remarkable cycle, named after French physicist Nicolas Léonard Sadi Carnot, showcases the fundamental principles governing energy conversion. Prepare to be amazed by its elegance and significance in advancing our understanding of thermodynamics and its practical applications in various fields.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


