The Carnot cycle, named after French engineer Sadi Carnot, is a fundamental concept in thermodynamics. It forms the basis for understanding how heat engines work and provides vital insights into the efficiency of such systems. The cycle consists of a series of four processes, including two reversible isothermal processes and two reversible adiabatic processes, that ultimately allow for the conversion of heat into work.
In this article, we will delve into the fascinating world of the Carnot cycle and explore 20 unbelievable facts that will blow your mind. From its historical significance to its practical applications, these facts will shed light on the unique features and properties of this thermodynamic cycle. So, fasten your seatbelts and get ready to embark on a journey through the intricate workings of the Carnot cycle!
Key Takeaways:
- The Carnot Cycle, named after physicist Sadi Carnot, is a super-efficient heat engine cycle that sets the bar for real-world engines. It’s like the gold standard of heat engines, showing how efficient they can be.
- The Carnot Cycle is like a superhero for thermodynamics, teaching us about maximum efficiency and the limitations of converting heat into useful work. It’s the blueprint for designing super-efficient systems.
The Carnot Cycle is named after French physicist Sadi Carnot.
Sadi Carnot is renowned for his work on the theory of heat engines and the establishment of the concept of an idealized heat engine known as the Carnot Cycle.
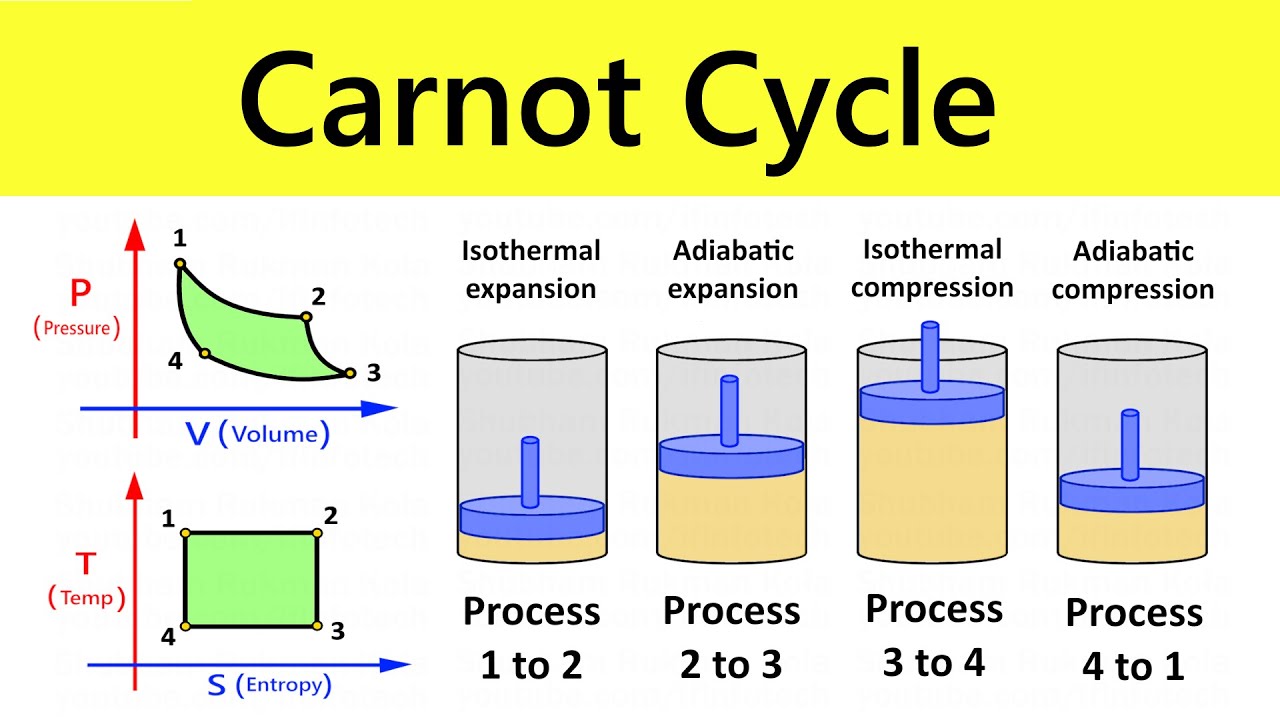
The Carnot Cycle is a thermodynamic cycle that operates between two heat reservoirs.
It consists of four processes: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression.
The Carnot Cycle is the most efficient heat engine cycle.
It achieves the highest possible efficiency for a heat engine operating between two specific temperatures.
Efficiency of the Carnot Cycle is determined by the temperature of the two reservoirs.
The higher the temperature of the hot reservoir and the lower the temperature of the cold reservoir, the higher the efficiency of the Carnot Cycle.
The Carnot Cycle is used as a benchmark to compare the performance of real heat engines.
It provides a theoretical upper limit on the efficiency that any real heat engine can achieve.
The Carnot Cycle is reversible.
It can be run in reverse to function as a heat pump, transferring heat from a colder reservoir to a hotter reservoir.
The Carnot Cycle is based on the principles of the second law of thermodynamics.
It demonstrates that heat cannot spontaneously flow from a colder body to a hotter body without the input of external work.
The Carnot Cycle is an idealized cycle and does not account for real-world friction and energy losses.
In practical applications, these losses reduce the efficiency of real heat engines compared to the ideal Carnot Cycle.
The Carnot Cycle can be represented on a pressure-volume (P-V) diagram.
The diagram shows the four processes of the cycle and the relationship between pressure and volume during each process.
The Carnot Cycle is commonly used in the analysis of power plants and refrigeration systems.
It provides a theoretical framework for understanding the efficiency and performance of these systems.
The Carnot Cycle is used to calculate the maximum possible efficiency of a heat engine.
By understanding the Carnot efficiency, engineers can optimize the design and operation of real heat engines.
The Carnot Cycle is based on the assumption of negligible heat transfer during adiabatic processes.
This assumption simplifies the calculations and allows for the determination of the maximum efficiency of the cycle.
The Carnot Cycle is a closed system.
It does not exchange matter with its surroundings during operation.
The Carnot Cycle can be used to model the behavior of gases and other thermodynamic systems.
Its principles can be applied to understand and predict the behavior of various systems in different fields of science and engineering.
The Carnot Cycle provides insights into the limitations of converting heat into useful work.
It demonstrates that not all heat energy can be converted into mechanical work due to the second law of thermodynamics.
The Carnot Cycle is an idealized representation of real-world heat engines.
It serves as a theoretical framework to analyze and improve the efficiency of practical heat engines.
The Carnot Cycle does not involve any chemical reactions.
It solely focuses on the transfer of heat energy between the reservoirs and the working fluid.
The Carnot Cycle can be applied to various processes, including power generation and refrigeration.
Its principles are utilized in industries to optimize energy usage and improve overall system performance.
The Carnot Cycle illustrates the importance of temperature differences in thermodynamic systems.
It emphasizes that a larger temperature difference leads to a higher efficiency of the cycle.
The Carnot Cycle plays a fundamental role in the field of thermodynamics and the understanding of heat engines.
It provides a cornerstone for analyzing and designing efficient thermodynamic systems.
Conclusion
The Carnot Cycle is a remarkable thermodynamic process that has revolutionized our understanding of heat engines and their efficiency. It is based on a series of reversible processes and provides insight into the maximum efficiency that can be achieved in any heat engine operating between two temperature reservoirs.
Understanding the various stages of the Carnot Cycle, including the isothermal and adiabatic processes, allows engineers to design more efficient engines and improve energy conversion systems. By maximizing the efficiency of heat engines, we can reduce our reliance on fossil fuels and move towards more sustainable energy sources.
As we continue to explore and understand the principles behind the Carnot Cycle, we unlock new possibilities for clean energy generation, transportation, and industrial processes. The remarkable facts and applications related to the Carnot Cycle open up a world of scientific and technological advancements that have the potential to transform our society.
FAQs
Q: What is the Carnot Cycle?
A: The Carnot Cycle is a theoretical thermodynamic cycle that represents the most efficient way to convert heat into work. It consists of four stages: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression.
Q: Who developed the Carnot Cycle?
A: The Carnot Cycle was developed by French physicist Sadi Carnot in 1824. His work laid the foundation for the field of thermodynamics and established the concept of maximum efficiency in heat engines.
Q: What is the significance of the Carnot Cycle?
A: The Carnot Cycle provides a theoretical upper limit on the efficiency of heat engines. It helps scientists and engineers understand the fundamental principles of energy conversion and guides the design of more efficient engines and systems.
Q: Can the Carnot Cycle be implemented in real-life systems?
A: While the Carnot Cycle is an idealized representation, its principles are applied in various practical systems. Heat pumps, refrigerators, and power plants strive to approach the maximum efficiency predicted by the Carnot Cycle.
Q: How does the Carnot Cycle contribute to sustainable energy?
A: By understanding and optimizing the Carnot Cycle, we can improve the efficiency of energy conversion systems. This leads to reduced energy waste, increased utilization of renewable energy sources, and a more sustainable energy future.
The Carnot Cycle, a fundamental concept in thermodynamics, reveals the limits of efficiency for heat engines and provides a foundation for understanding pressure and temperature relationships. Dive deeper into the fascinating world of thermodynamics by exploring intriguing facts about how heat engines convert thermal energy into mechanical work, and captivating facts about pressure, a crucial factor in many physical systems. Uncover the secrets behind these essential concepts and gain a deeper appreciation for the intricate workings of our universe.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
