Iodic acid might sound like something straight out of a chemistry lab, but it's more common than you think. This powerful compound, with the formula HIO3, plays a crucial role in various industries and scientific research. Ever wondered what makes iodic acid so special? Iodic acid is known for its strong oxidizing properties, making it a key player in chemical reactions. It's also used in the production of iodine, a vital element for human health. From its role in analytical chemistry to its presence in everyday products, iodic acid is a fascinating substance worth exploring. Ready to dive into the world of iodic acid? Let's uncover 50 intriguing facts about this remarkable compound!
Key Takeaways:
- Iodic acid, a powerful chemical compound, has diverse applications in industry and chemistry. It's crucial to handle it with care due to its strong oxidizing nature and potential environmental impact.
- Discovered in 1813, iodic acid continues to be a subject of modern research, offering potential in green chemistry, pharmaceutical synthesis, and nanotechnology. Its future looks promising with ongoing exploration into new applications.
What is Iodic Acid?
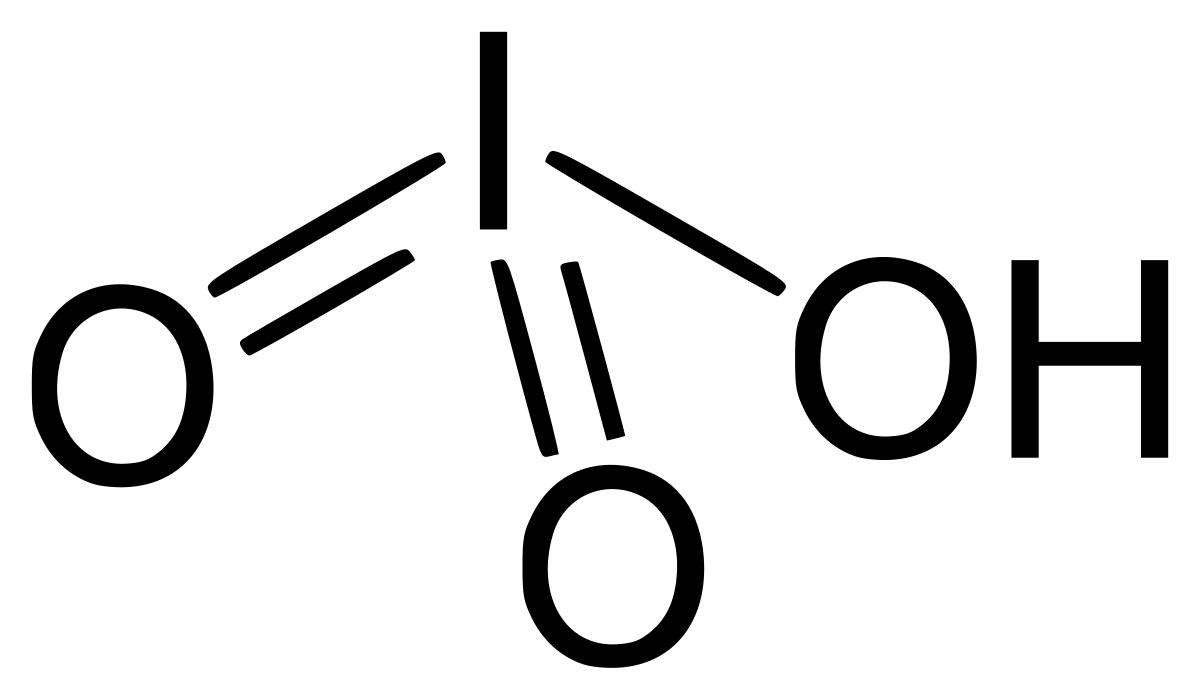
Iodic acid is a chemical compound with the formula HIO₃. It is a white, crystalline solid that is highly soluble in water. This compound is an important oxidizing agent and has various applications in chemistry and industry.
- Iodic acid has the chemical formula HIO₃.
- It appears as a white, crystalline solid.
- Highly soluble in water, making it easy to use in aqueous solutions.
- Acts as a strong oxidizing agent.
- Commonly used in analytical chemistry for titrations.
Chemical Properties of Iodic Acid
Understanding the chemical properties of iodic acid helps in its application across different fields. Here are some key chemical properties:
- Iodic acid is a strong acid.
- It dissociates completely in water to form hydrogen ions (H⁺) and iodate ions (IO₃⁻).
- The pKa value of iodic acid is approximately 0.75.
- It can oxidize various substances, including metals and organic compounds.
- Iodic acid can be reduced to iodine (I₂) under certain conditions.
Uses of Iodic Acid
Iodic acid has numerous applications due to its strong oxidizing properties. Here are some of its uses:
- Used in the synthesis of iodine compounds.
- Employed in analytical chemistry for redox titrations.
- Acts as a reagent in the determination of certain metals.
- Utilized in the production of disinfectants.
- Plays a role in the manufacturing of pharmaceuticals.
Safety and Handling of Iodic Acid
Handling iodic acid requires caution due to its strong oxidizing nature. Here are some safety tips:
- Iodic acid can cause severe skin burns.
- It is harmful if inhaled or ingested.
- Always wear protective gear, including gloves and goggles, when handling.
- Store in a cool, dry place away from incompatible substances.
- In case of contact with skin or eyes, rinse immediately with plenty of water.
Environmental Impact of Iodic Acid
The environmental impact of iodic acid is an important consideration. Here are some facts about its effects on the environment:
- Iodic acid can contribute to water pollution if not disposed of properly.
- It can oxidize organic matter in water bodies, affecting aquatic life.
- Proper disposal methods are essential to minimize environmental impact.
- It is not considered a persistent organic pollutant.
- Regulatory guidelines exist for the safe disposal of iodic acid.
Historical Context of Iodic Acid
Iodic acid has a rich history in the field of chemistry. Here are some historical facts:
- Discovered by the French chemist Joseph Louis Gay-Lussac in 1813.
- Initially used in the study of iodine and its compounds.
- Played a role in early research on oxidation-reduction reactions.
- Contributed to the development of modern analytical chemistry techniques.
- Its discovery helped advance the understanding of halogen chemistry.
Iodic Acid in Modern Research
Iodic acid continues to be a subject of research in various scientific fields. Here are some current research areas:
- Studied for its potential use in green chemistry.
- Investigated for its role in atmospheric chemistry.
- Used in the development of new analytical methods.
- Explored for its potential in pharmaceutical synthesis.
- Research ongoing into its applications in nanotechnology.
Fun Facts about Iodic Acid
Here are some interesting and lesser-known facts about iodic acid:
- Iodic acid can form hydrates with water.
- It can be used to prepare iodates, which are salts of iodic acid.
- Iodic acid can act as a catalyst in certain chemical reactions.
- It has a melting point of 110°C (230°F).
- Iodic acid can be used to test for the presence of starch.
Iodic Acid in Everyday Life
Though not commonly encountered in daily life, iodic acid has some everyday applications:
- Used in some water purification processes.
- Plays a role in the production of certain food additives.
- Can be found in some cleaning agents.
- Used in laboratories for educational purposes.
- Sometimes used in the preparation of iodized salt.
Future Prospects of Iodic Acid
The future of iodic acid looks promising with ongoing research and potential new applications:
- Potential use in renewable energy technologies.
- Exploration of its role in sustainable chemical processes.
- Development of new industrial applications.
- Continued research into its environmental impact.
- Potential for new medical and pharmaceutical uses.
The Final Word on Iodic Acid
Iodic acid, a fascinating compound, plays a crucial role in various fields. From its use in chemical reactions to its importance in medical applications, it’s clear why this substance garners attention. Understanding its properties, like being a strong oxidizing agent and its solubility in water, helps us appreciate its versatility.
Whether you’re a student, a professional, or just curious, knowing these 50 facts about iodic acid enriches your knowledge. It’s not just about the science; it’s about seeing how such compounds impact our daily lives.
Keep exploring, keep learning, and remember, every fact adds a layer to your understanding of the world. Iodic acid is just one piece of the vast puzzle of chemistry, but it’s a piece worth knowing.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
