Barium Peroxide might sound like a complex chemical, but it's actually quite fascinating! This compound, with the formula BaO2, has a variety of uses and interesting properties. Did you know it plays a crucial role in the production of hydrogen peroxide? Barium Peroxide is also used in fireworks to create brilliant green colors. Beyond that, it has historical significance in the development of early oxygen masks for pilots. Whether you're a chemistry enthusiast or just curious, these 40 facts about Barium Peroxide will surely spark your interest and expand your knowledge about this intriguing substance.
Key Takeaways:
- Barium Peroxide, with the formula BaO₂, is a powerful oxidizing agent used in fireworks, glass manufacturing, and pharmaceuticals. It's toxic and requires careful handling to avoid health risks.
- When handling Barium Peroxide, always wear protective gear, ensure proper ventilation, and follow safety guidelines for storage, disposal, and spill management. It's important to prioritize safety to avoid accidents and health issues.
What is Barium Peroxide?
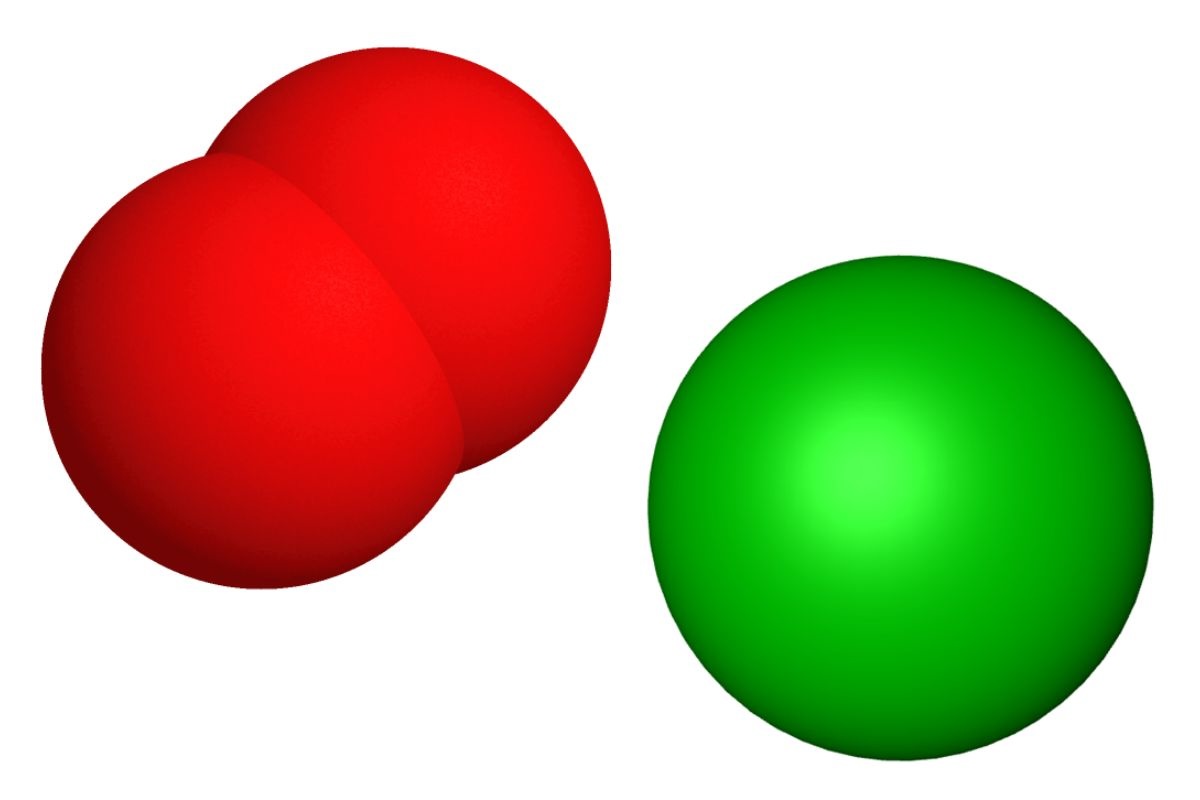
Barium Peroxide, a chemical compound with the formula BaO₂, is known for its strong oxidizing properties. It has various applications in industries and laboratories. Here are some intriguing facts about this compound.
-
Chemical Formula: Barium Peroxide's chemical formula is BaO₂, indicating it contains one barium atom and two oxygen atoms.
-
Appearance: It appears as a grayish-white solid, often found in powder form.
-
Oxidizing Agent: This compound is a powerful oxidizing agent, making it useful in many chemical reactions.
-
Discovery: Barium Peroxide was discovered in the early 19th century by Johann Ritter, a German chemist.
-
Molecular Weight: The molecular weight of Barium Peroxide is approximately 169.34 g/mol.
-
Solubility: It is slightly soluble in water but dissolves more readily in acids.
-
Decomposition: When heated, Barium Peroxide decomposes to barium oxide (BaO) and oxygen gas (O₂).
-
Uses in Pyrotechnics: This compound is used in fireworks and flares to produce green colors.
-
Historical Use: Historically, it was used to produce hydrogen peroxide (H₂O₂) before modern methods were developed.
-
Safety Concerns: Barium Peroxide is toxic if ingested or inhaled, requiring careful handling.
Industrial Applications of Barium Peroxide
Barium Peroxide has several industrial applications due to its unique properties. Let's explore some of these uses.
-
Glass Manufacturing: It is used in the production of certain types of glass to enhance clarity and strength.
-
Bleaching Agent: Barium Peroxide acts as a bleaching agent in the textile industry.
-
Welding: It is used in welding and cutting metals as an oxygen source.
-
Rubber Industry: This compound is used in the rubber industry to improve the quality of rubber products.
-
Oxidation Reactions: Barium Peroxide is employed in various oxidation reactions in chemical manufacturing.
-
Catalyst: It serves as a catalyst in some chemical processes, speeding up reactions.
-
Paper Industry: This compound is used in the paper industry for bleaching paper pulp.
-
Pharmaceuticals: It has applications in the pharmaceutical industry for synthesizing certain drugs.
-
Ceramics: Barium Peroxide is used in the ceramics industry to produce specific types of ceramics.
-
Electronics: It is used in the electronics industry for manufacturing certain electronic components.
Chemical Properties of Barium Peroxide
Understanding the chemical properties of Barium Peroxide helps in grasping its behavior in various reactions.
-
Oxidation State: Barium in Barium Peroxide has an oxidation state of +2, while oxygen has an oxidation state of -1.
-
Reactivity: It reacts vigorously with water, acids, and organic materials.
-
Thermal Stability: Barium Peroxide is thermally stable up to a certain temperature, beyond which it decomposes.
-
pH Level: In aqueous solutions, it tends to create a basic environment.
-
Redox Reactions: It participates in redox reactions, acting as an oxidizing agent.
-
Hydrogen Peroxide Production: Historically, it was used to produce hydrogen peroxide through a reaction with sulfuric acid.
-
Reaction with Acids: When reacted with acids, it releases oxygen gas.
-
Combustion: It supports combustion by releasing oxygen, making it useful in pyrotechnics.
-
Incompatibility: Barium Peroxide is incompatible with reducing agents, organic materials, and combustible substances.
-
Storage: It should be stored in a cool, dry place away from incompatible materials.
Safety and Handling of Barium Peroxide
Handling Barium Peroxide requires caution due to its toxic and reactive nature. Here are some safety tips.
-
Protective Gear: Always wear protective gear, including gloves and goggles, when handling Barium Peroxide.
-
Ventilation: Ensure proper ventilation in the area where it is being used to avoid inhalation of fumes.
-
First Aid: In case of contact with skin or eyes, rinse immediately with plenty of water and seek medical attention.
-
Storage: Store in a tightly sealed container in a cool, dry place away from incompatible substances.
-
Disposal: Dispose of Barium Peroxide according to local regulations, as it is considered hazardous waste.
-
Spill Management: In case of a spill, avoid creating dust and clean up using appropriate protective equipment.
-
Fire Hazards: Keep away from heat sources and open flames, as it can support combustion.
-
Inhalation Risk: Avoid inhaling dust or fumes, as it can cause respiratory issues.
-
Ingestion Risk: Do not ingest, as it is toxic and can cause severe health problems.
-
Emergency Procedures: Have emergency procedures in place, including access to eyewash stations and safety showers.
Barium Peroxide: The Final Word
Barium peroxide, a compound with the formula BaO2, has a rich history and diverse uses. From its role in fireworks to its application in oxygen generation, this chemical has proven its versatility. It's fascinating how a single compound can be so integral in various fields, including pyrotechnics, medicine, and even environmental science.
Understanding barium peroxide's properties, like its ability to release oxygen, helps us appreciate its importance. This compound isn't just a scientific curiosity; it's a practical tool in many industries. Whether you're a student, a professional, or just curious, knowing these facts can broaden your perspective on how chemicals impact our daily lives.
So next time you see a dazzling firework display or hear about oxygen therapy, remember the role barium peroxide plays. It's a small but mighty player in the world of chemistry.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
