The world of chemistry is a vast and fascinating one, filled with countless phenomena and processes that shape the natural world around us. One such process that plays a crucial role in many chemical reactions is redox, short for reduction-oxidation. Redox reactions involve the transfer of electrons between molecules, leading to remarkable transformations and resulting in various forms of matter. Understanding redox is crucial not only in chemistry but also in fields such as biology, environmental science, and medicine. In this article, we will explore 20 extraordinary facts about redox, shedding light on its importance and the intriguing ways it manifests in our everyday lives. From its role in energy production to its significance in metal corrosion, redox is a key concept that unveils the wonders of chemical phenomena. So, let us dive into the realm of redox and uncover the secrets it holds!
Key Takeaways:
- Redox is a fascinating chemical process that powers our bodies, fuels batteries, and drives environmental processes. It’s like the superhero of chemistry, making amazing things happen in the world around us!
- Redox reactions are like a dynamic dance between molecules, balancing the scales of chemical transformations and playing a crucial role in everything from medicine to environmental protection. It’s like the secret ingredient that makes the world go round!
Redox: The Dynamic Chemistry Phenomenon
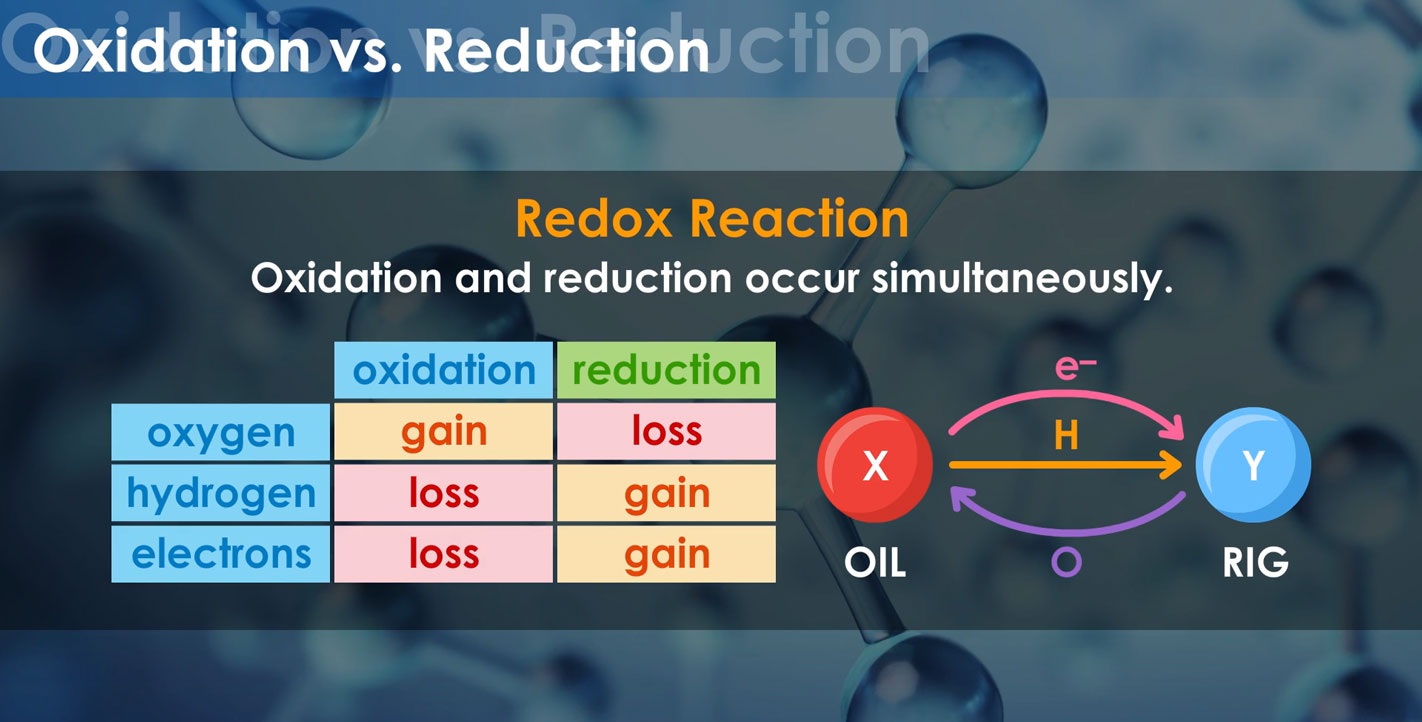
Redox, short for reduction-oxidation, is a fascinating chemical process that involves the transfer of electrons between molecules. It plays a crucial role in numerous natural and artificial reactions.
Redox: Balancing the Scale of Chemical Transformations
One of the key functions of redox reactions is their ability to balance the scales of chemical transformations by ensuring that the number of electrons lost in oxidation matches the number gained in reduction.
Redox: Enabling the Energy Production in Our Bodies
The process of redox is vital in cellular respiration, where it facilitates the production of energy by oxidizing glucose molecules to produce carbon dioxide, water, and adenosine triphosphate (ATP).
Redox: Fueling the Power of Batteries
Redox reactions are at the heart of battery technology, allowing the storage and conversion of chemical energy into electrical energy through the transfer of electrons.
Redox: Driving the Photosynthesis Process
In photosynthesis, redox reactions are responsible for the conversion of solar energy into chemical energy. During this process, water molecules are oxidized to release oxygen, while carbon dioxide is reduced to synthesize glucose.
Redox: Indispensable in Chemical Industry
Redox reactions find widespread use in the chemical industry, serving as key tools for the production of various chemicals, including acids, metals, and synthetic compounds.
Redox: Unleashing the Power of Combustion
Combustion, the process of burning, is a classic redox reaction wherein a fuel substance combines rapidly with oxygen to release energy in the form of heat and light.
Redox: Corrosion and Rusting
Redox reactions are responsible for the corrosion and rusting of metals when exposed to oxygen and moisture. In these processes, the metal undergoes oxidation, leading to the formation of metal oxides.
Redox: Biological Antioxidant Defense
Living organisms employ redox reactions as a defense mechanism against oxidative stress by using antioxidants to neutralize harmful reactive oxygen species (ROS) and maintain the balance of redox processes within cells.
Redox: Essential for Environmental Processes
Redox reactions play a critical role in environmental processes, such as the nitrogen cycle, sulfur cycle, and carbon cycle, influencing the balance of nutrients and the overall health of ecosystems.
Redox: Generating Electricity Through Fuel Cells
Fuel cells utilize redox reactions to produce electricity directly from chemical reactions, with hydrogen fuel being oxidized at the anode and oxygen being reduced at the cathode.
Redox: Medicine and Pharmaceuticals
Redox reactions have significant applications in medicine and pharmaceuticals, including drug metabolism, the body’s defense against pathogens, and the development of novel therapeutic agents.
Redox: Oxidation and Reduction Potentials
The concept of oxidation-reduction potentials allows scientists to measure the tendency of a substance to gain or lose electrons and helps predict the direction and feasibility of redox reactions.
Redox: Environmental Remediation
Redox reactions play a vital role in environmental remediation to remove pollutants and toxins from soil and water. By utilizing specific redox reactions, contaminants can be transformed into less hazardous substances.
Redox: Estimating Age with Radiocarbon Dating
Radiocarbon dating is based on the principle of redox reactions. By measuring the ratio of carbon isotopes, scientists can determine the age of organic materials with remarkable accuracy.
Redox: Electroplating and Metal Deposition
Electroplating is an application of redox reactions that involves depositing a layer of metal onto an object’s surface, enhancing its appearance and providing corrosion resistance.
Redox: Reducing Agents and Oxidizing Agents
Redox reactions involve two key players – reducing agents and oxidizing agents. Reducing agents donate electrons, while oxidizing agents accept electrons, allowing for electron transfer in redox processes.
Redox: Environmental Significance of Ozone
Ozone, a vital component of the Earth’s atmosphere, is formed through redox reactions involving oxygen molecules and oxygen atoms. It plays a critical role in protecting the planet from harmful ultraviolet radiation.
Redox: Electron Transport Chain in Cellular Respiration
The electron transport chain, a series of redox reactions, is a crucial step in cellular respiration, producing ATP and powering various cellular processes.
Redox: Bridging the Gap Between Chemistry and Biology
Redox reactions are fundamental to understanding the vital intersection between chemistry and biology, shaping the processes of life and influencing the development of new medical treatments and technologies.
Conclusion
In conclusion, redox reactions are fascinating and essential processes in chemistry. They play a crucial role in various aspects of our daily lives, from energy production to industrial processes. Understanding the principles of redox reactions is vital for comprehending the world around us.
Through this article, we have explored 20 extraordinary facts about redox. From the importance of oxidation and reduction to the application of redox reactions in different fields, we have seen how these reactions impact our world in numerous ways.
Whether you’re a chemistry enthusiast or simply curious about the fundamental principles that govern chemical reactions, the world of redox provides a captivating journey into the intricacies of chemistry. So, keep exploring, keep learning, and dive deeper into the captivating world of redox reactions!
FAQs
1. What is a redox reaction?
A redox reaction, short for oxidation-reduction reaction, involves the transfer of electrons between two reactants. One substance undergoes oxidation (loses electrons), while another substance simultaneously undergoes reduction (gains electrons).
2. How are redox reactions essential in everyday life?
Redox reactions are crucial in various aspects of our daily lives. They are involved in energy production, such as in the combustion of fuels. They also play a significant role in corrosion, digestion, photosynthesis, and various industrial processes.
3. What are some examples of redox reactions?
Examples of redox reactions include the rusting of iron, the burning of gasoline, the digestion of food, the respiration process in living organisms, and the photosynthesis process in plants.
4. How can redox reactions be balanced?
To balance a redox reaction, one must ensure the conservation of both mass and charge. This can be achieved by adjusting the coefficients of the reactants and products, as well as balancing the number of electrons transferred between the species involved in the reaction.
5. How do redox reactions relate to the concept of oxidation numbers?
Oxidation numbers are assigned to elements in compounds or ions to track electron transfer in redox reactions. The increase in oxidation number indicates oxidation, and the decrease indicates reduction.
6. Are redox reactions reversible?
Yes, redox reactions can be reversible. Some redox reactions can proceed in both forward and reverse directions, depending on the conditions and concentrations of reactants and products.
7. What is the significance of redox reactions in environmental processes?
Redox reactions impact various environmental processes, including the degradation of pollutants and the cycling of elements in ecosystems. Understanding redox reactions is crucial in environmental chemistry and pollution control.
Redox reactions play a crucial role in our world, from powering batteries to driving photosynthesis. Understanding the principles behind these chemical transformations opens doors to fascinating aspects of chemistry. Curious minds can explore the enigmatic nature of half-reactions, delve into the astonishing complexities of redox reactions, or unravel the mindblowing intricacies of redox titration. Each topic offers a unique perspective on the captivating world of redox chemistry, inviting readers to expand their knowledge and appreciate the marvels of these fundamental processes. Embark on a journey of discovery and uncover the secrets that redox holds.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
