The world of chemistry is full of fascinating phenomena that exist at the microscopic level. One such phenomenon that has puzzled scientists for decades is the ion-dipole interaction. This complex interaction involves charged particles, known as ions, and polar molecules, forming unique bonds that have significant implications in various chemical processes. Understanding the intricacies of ion-dipole interactions is crucial for comprehending a wide range of chemical reactions, including solvation, electrolyte solutions, and even some biological processes.
In this article, we will delve into the enigmatic world of ion-dipole interactions and explore 20 intriguing facts about this phenomenon. From the role of electrostatic forces to the applications in chemicals and everyday life, we will uncover the secrets behind these unique interactions. So, let’s embark on a journey through the mysterious world of ion-dipole interactions and unlock the science behind their fascinating existence.
Key Takeaways:
- Ion-dipole interactions are powerful forces that bring ions and polar molecules together, influencing everything from dissolving salts in water to the stability of crystal structures. They’re like magnets for molecules, shaping the world of chemistry and materials science.
- Understanding ion-dipole interactions is like having a secret code to unlock the mysteries of chemical behavior and create new materials. It’s like learning the language of molecules, opening doors to endless possibilities in science and technology.
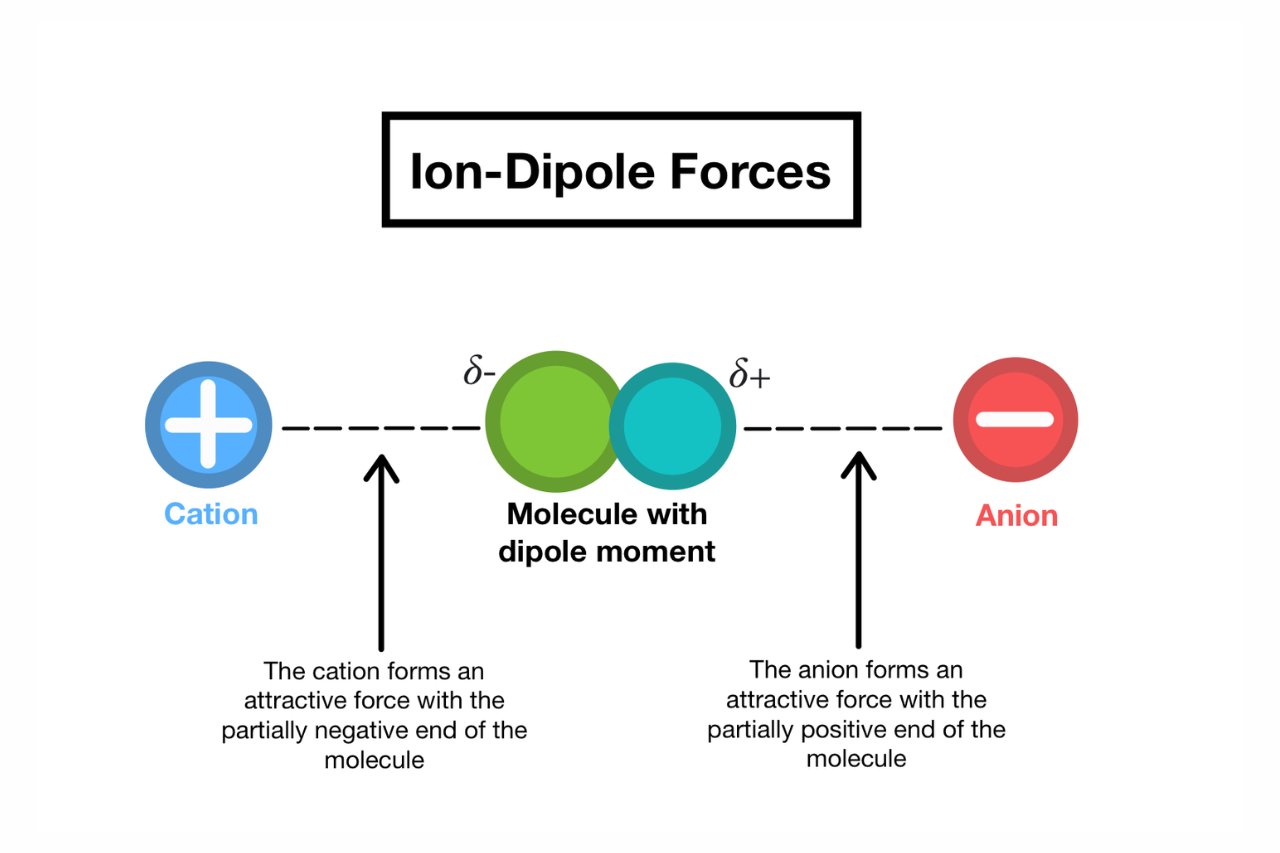
Ion-dipole interactions are electrostatic attractions between ions and polar molecules.
These interactions occur due to the difference in charge distribution between the positive or negative ions and the polar molecules.
Ion-dipole interactions are the strongest type of intermolecular forces.
Compared to other intermolecular forces such as hydrogen bonding, van der Waals forces, or dipole-dipole interactions, ion-dipole interactions exhibit the highest level of attraction.
Ion-dipole interactions are responsible for dissolving salts in water.
Water molecules form a hydration shell around the ions, enabling them to dissociate and dissolve in the solvent.
Ion-dipole interactions play a crucial role in biochemical processes.
They facilitate the binding of ions to proteins, enabling the functioning of enzymes and other biological molecules.
Ion-dipole interactions can influence the physical properties of solvents.
The strength of ion-dipole interactions affects properties such as boiling point, melting point, and viscosity of solvents.
Ion-dipole interactions are essential in electrolyte solutions.
They promote the conductivity of solutions by allowing the movement of ions.
Ion-dipole interactions can affect the outcome of chemical reactions.
Depending on the strength of the ion-dipole interactions, the rate and equilibrium of reactions can be altered.
Ion-dipole interactions can be manipulated in materials science.
By designing materials with specific ion-dipole interactions, properties such as magnetism, conductivity, and optical behavior can be tailored.
Ion-dipole interactions are influenced by the size and charge of ions.
Larger ions with higher charges tend to have stronger ion-dipole interactions due to increased electrostatic forces.
Ion-dipole interactions can affect the solubility of compounds.
Compounds with stronger ion-dipole interactions are generally more soluble in polar solvents.
Ion-dipole interactions can lead to the formation of ion-dipole complexes.
These complexes involve the close association of ions and polar molecules, resulting in unique structural arrangements.
Ion-dipole interactions are essential in the purification of water.
In processes like reverse osmosis, ion-dipole interactions help remove impurities and ensure clean drinking water.
Ion-dipole interactions can affect the solubility of gases in liquids.
The presence of ions in a solvent can enhance or hinder the dissolution of gases.
Ion-dipole interactions are relevant in the field of electrochemistry.
They govern processes such as electrode reactions, ion transport, and electrolysis.
Ion-dipole interactions can influence the stability of crystal structures.
The arrangement of ions and polar molecules in a crystal lattice is determined by the strength and directionality of ion-dipole interactions.
Ion-dipole interactions can occur in the gas phase.
Even in the absence of a solvent, ions and polar molecules can attract each other due to their opposite charges.
Ion-dipole interactions can be used to separate mixtures.
Techniques like ion exchange chromatography exploit the selective interactions between ions and polar stationary phases.
Ion-dipole interactions can influence the pH of solutions.
The presence of ions and their interactions with water molecules can affect the acidity or basicity of a solution.
Ion-dipole interactions are involved in the formation of hydrogen bonds.
Hydrogen bonding, which is a type of ion-dipole interaction, occurs between hydrogen atoms in polar molecules and electronegative atoms like oxygen or nitrogen.
Ion-dipole interactions are widely studied in physical chemistry and materials science.
Researchers continue to explore the intricacies of these interactions to improve our understanding of molecular behavior and develop innovative materials.
These 20 enigmatic facts about ion-dipole interactions shed light on the importance of these interactions in various scientific disciplines. Whether you are studying chemistry, biochemistry, materials science, or any related field, understanding ion-dipole interactions is crucial for unlocking the mysteries of chemical behavior and designing new technologies.
Conclusion
In conclusion, ion-dipole interactions are fascinating phenomena that play a crucial role in many aspects of chemistry. These interactions occur when ions and polar molecules come together and create a connection based on their opposite charges. Through these interactions, ions and dipolar molecules form strong bonds, effectively dissolving and allowing for the transport of charged particles in solutions. Understanding ion-dipole interactions is of great significance in fields such as electrochemistry, biochemistry, and materials science.
FAQs
Q: What are ion-dipole interactions?
A: Ion-dipole interactions occur when charged ions and polar molecules interact and form bonds due to their opposite charges. These interactions play a crucial role in various chemical processes and solutions.
Q: What is the importance of ion-dipole interactions?
A: Ion-dipole interactions are essential for understanding the behavior of ions in solution and the ability of polar solvents to dissolve ionic compounds. They are also significant in numerous processes such as ionic conductivity, acid-base reactions, and many biological reactions.
Q: How do ion-dipole interactions influence the properties of solutions?
A: Ion-dipole interactions affect properties such as boiling point, freezing point, and viscosity of solutions. These interactions provide a driving force for the dissolution of ionic compounds and influence the electrostatic environment in the solution.
Q: Can you provide an example of ion-dipole interactions in daily life?
A: One example of ion-dipole interactions is when table salt (sodium chloride) dissolves in water. The positive sodium ions attract the negative oxygen ends of water molecules, while the negative chloride ions attract the positive hydrogen ends, creating a solution.
Q: How are ion-dipole interactions different from other types of intermolecular forces?
A: Ion-dipole interactions are different from other intermolecular forces such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces because they involve ions and polar molecules specifically. These interactions are stronger than most other intermolecular forces.
Unraveling the enigmatic world of ion-dipole interactions is just the beginning. Chemical bonds form the foundation of matter, and their incredible properties will leave you in awe. Physical chemistry holds the key to understanding the fundamental principles governing the behavior of substances. Dive deeper into the fascinating realm of molecular interactions, where the lock-and-key model elegantly explains the specificity of biochemical processes.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
