Key Takeaways:
- The Shielding Effect in chemistry repels outer electrons from the nucleus, impacting atomic size, ionization energy, and chemical reactivity. It’s like a force field shaping how atoms interact and bond.
- Shielding affects the behavior of elements in the periodic table, influencing their reactivity and ability to form chemical bonds. It’s like a secret ingredient that determines how atoms behave in the world of chemistry.
The Shielding Effect Defined
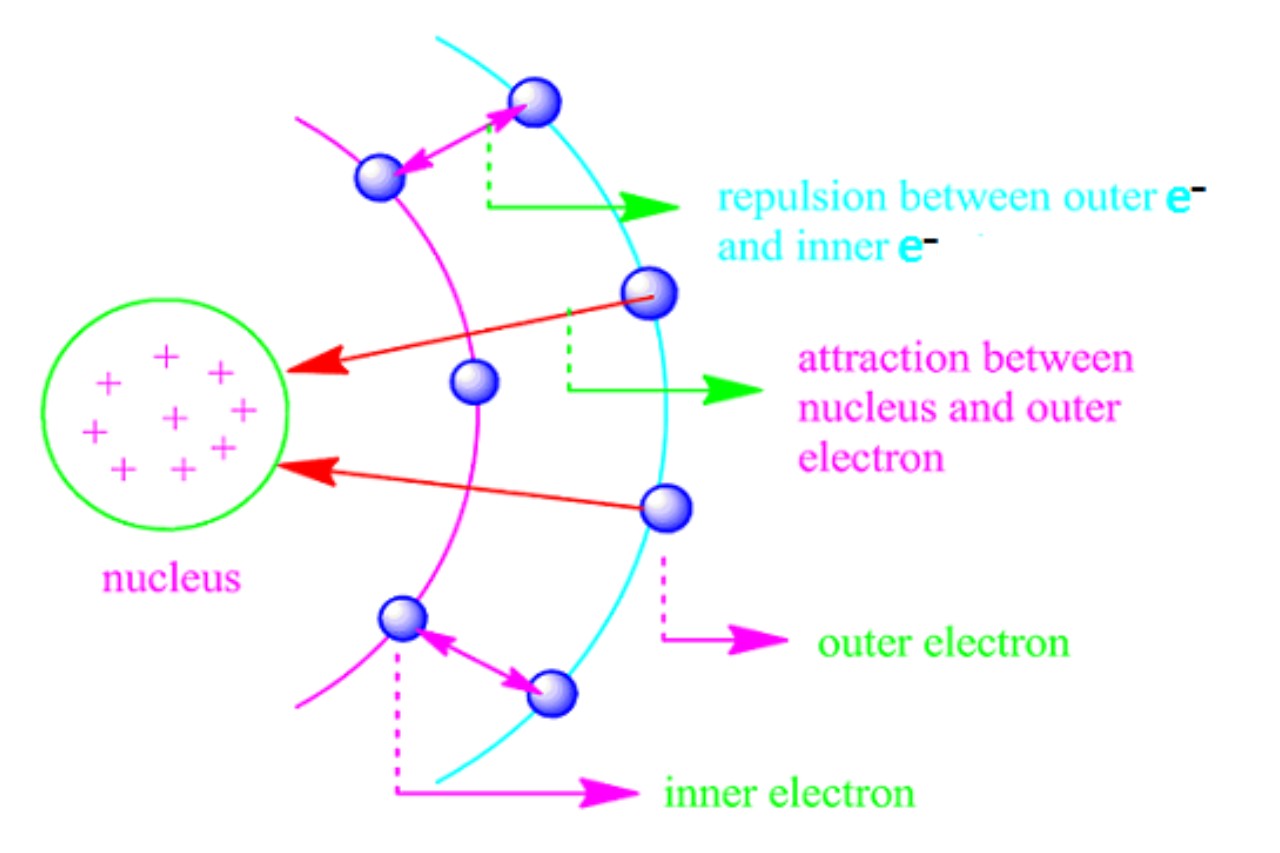
The shielding effect is a fundamental concept in chemistry that refers to the phenomenon where inner electrons in an atom “shield” or repel outer electrons from the full charge of the atom’s nucleus.
Electronegativity and Shielding Effect
The shielding effect plays a crucial role in determining the electronegativity of an atom. Elements with effective shielding have lower electronegativity values, making them less likely to attract electrons in a chemical bond.
Effective Nuclear Charge
The shielding effect reduces the effective nuclear charge experienced by outer electrons. The effective nuclear charge is the net positive charge felt by an electron, determined by subtracting the shielding effect from the actual nuclear charge.
Periodic Trend
The shielding effect increases down a group in the periodic table. This is due to the addition of new energy levels and increased distance between the nucleus and the outer electrons, resulting in greater electron-electron repulsion.
Atomic Radius and Shielding Effect
The shielding effect contributes to the increase in atomic radius as you move down a group. The outer electrons are shielded from the full charge of the nucleus, allowing them to occupy larger orbitals and thus increasing the size of the atom.
Shielding and Ionization Energy
The shielding effect reduces the ionization energy of an atom. It becomes easier to remove an electron from an atom with effective shielding because the outer electron is less strongly attracted to the positively charged nucleus.
Shielding Effect and Periodic Table Groups
The shielding effect is responsible for the observed trend in ionization energy within a group. As you move down a group, the shielding effect increases, resulting in lower ionization energy values due to the decreased attraction between the nucleus and the outer electrons.
Shielding Effect and Chemical Bonds
The shielding effect influences the strength and nature of chemical bonds. Elements with greater shielding tend to form weaker bonds since the outer electrons are less strongly attracted to the nucleus.
Shielding Effect and Reactivity
The shielding effect affects the reactivity of elements. Elements with strong shielding are generally more reactive since their outer electrons are not as tightly held and are more available for chemical reactions.
Shielding Effect and Periodic Table Trends
The shielding effect is essential in explaining several periodic table trends, including atomic size, ionization energy, and electronegativity.
Slater’s Rules
Slater’s rules are used to estimate the effective nuclear charge and the shielding effect in multi-electron atoms. These rules take into account various factors such as the penetration of electron orbitals and the shielding contributions of different electron groups.
Shielding Effect and Transition Metals
Transition metals have complex electron configurations and exhibit unique shielding effects. The presence of partially filled d orbitals influences the shielding experienced by outer electrons in transition metal atoms.
Shielding Effect in Organic Chemistry
The shielding effect is relevant in organic chemistry, particularly in NMR spectroscopy. It influences the chemical shift values observed in the NMR spectra of organic compounds, providing valuable information about the electron distribution in a molecule.
Influence of Shielding on Hybridization
The shielding effect influences the hybridization of orbitals in covalent compounds. It determines the extent to which atomic orbitals mix to form hybrid orbitals, which in turn affects the shape and geometry of molecules.
Shielding Effect and Periodic Table Blocks
The shielding effect varies among different blocks of the periodic table. The inner transition metals, also known as f-block elements, exhibit significant shielding effects due to the presence of inner 4f or 5f electrons.
Shielding Effect and Electron Configuration
The shielding effect is influenced by the electron configuration of an element. The distribution of electrons in different energy levels determines the extent of electron-electron repulsion and thus the strength of the shielding effect.
Shielding Effect and Chemical Properties
The shielding effect plays a vital role in determining the chemical properties of elements. It affects factors such as atomic radii, ionization energy, electronegativity, and the ability to form chemical bonds.
Shielding Effect and Periodic Table Periods
The shielding effect remains relatively constant within a period of the periodic table. While the number of protons in the nucleus increases, so does the number of inner electrons, resulting in a balanced shielding effect.
Shielding Effect and Noble Gases
Noble gases have full valence electron shells, resulting in strong shielding effects. This accounts for their low reactivity and stable nature, as their outer electrons are shielded from interactions with other atoms.
Shielding Effect and Chemical Bond Strength
The shielding effect influences the strength of chemical bonds. Elements with weaker shielding have stronger bonds, as the outer electrons are more strongly attracted to the nucleus and less available for bonding with other atoms.
The 20 astonishing facts about the shielding effect demonstrate its significance in understanding various chemical properties and periodic trends. From its impact on atomic size to its influence on ionization energy and reactivity, the shielding effect plays a crucial role in determining the behavior of elements across the periodic table. By repelling outer electrons from the nucleus, it shapes the intricacies of bonding, hybridization, and the overall reactivity of atoms. Shielding effects vary depending on factors such as electron configuration, periodic table blocks, and the specific elements under consideration.
In conclusion, the shielding effect is a fundamental concept in chemistry that helps explain the behavior of elements and their involvement in chemical reactions. It is a key aspect of understanding the interactions between electrons and the nucleus within an atom. By delving into the fascinating world of the shielding effect, we gain valuable insights into the intricate nature of atomic structure and the periodic table as a whole.
Conclusion
In conclusion, the concept of shielding effect in chemistry is an intriguing phenomenon that plays a significant role in determining the chemical behavior and properties of atoms and molecules. It refers to the ability of inner electrons to shield outer electrons from the full nuclear charge, thereby impacting the effective nuclear charge experienced by the outer electrons.Through the shielding effect, elements with more inner electron shells experience a greater degree of electron-electron repulsion, which weakens the attractive force between the nucleus and outer electrons. This leads to larger atomic radii, decreased ionization energy, and increased electron affinity.Understanding the shielding effect is crucial in many areas of chemistry, including the study of atomic structure, chemical bonding, and predicting the reactivity and properties of elements. It provides insights into trends within the periodic table and helps explain why certain elements exhibit unique characteristics.Overall, the shielding effect is a fascinating aspect of chemistry that highlights the intricate interactions between electrons and their surrounding environment. Further research and exploration in this field will undoubtedly contribute to our understanding of the fundamental principles governing the behavior of matter.
FAQs
1. What is shielding effect?
The shielding effect refers to the ability of inner electrons to shield outer electrons from the full nuclear charge.
2. How does shielding effect impact atomic radii?
The shielding effect leads to larger atomic radii as a result of the weakened attractive force between the nucleus and outer electrons.
3. What is the relationship between shielding effect and ionization energy?
The shielding effect decreases the effective nuclear charge felt by the outer electrons, thereby reducing the ionization energy.
4. How does the shielding effect affect electron affinity?
The shielding effect increases the electron affinity as a result of the weakened attractive force between the nucleus and incoming electrons.
5. Why is understanding the shielding effect important in chemistry?
Understanding the shielding effect is crucial in predicting the reactivity and properties of elements, as well as explaining trends within the periodic table.
6. Are there any exceptions to the shielding effect?
Yes, there are exceptions where the shielding effect may not follow the usual trends. This can occur in cases involving transition metals or elements with anomalous electron configurations.
7. Can the shielding effect be observed experimentally?
No, the shielding effect cannot be directly observed. It is a theoretical concept used to explain and predict the behavior of atoms and molecules.
8. How does the shielding effect influence chemical bonding?
The shielding effect affects the ease with which atoms can form chemical bonds. It determines the attraction between electrons from different atoms and influences the stability and strength of chemical compounds.
9. Are there any practical applications of the shielding effect?
Yes, the understanding of shielding effect is important in areas such as materials science, pharmaceutical research, and catalysis, where the manipulation of atomic and molecular interactions is crucial.
10. Can the shielding effect vary within the same group of the periodic table?
Yes, the shielding effect can vary within the same group due to differences in the number of inner electron shells and electron configurations of the elements.
Shielding effect plays a crucial role in atomic structures and chemical reactions. Understanding its impact can help you grasp the intricacies of chemistry. If you're curious about how effective nuclear charge influences shielding effect, our article "11 Captivating Facts About Effective Nuclear Charge" is a must-read. Dive into the fascinating world of chemistry and expand your knowledge with our engaging content.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
