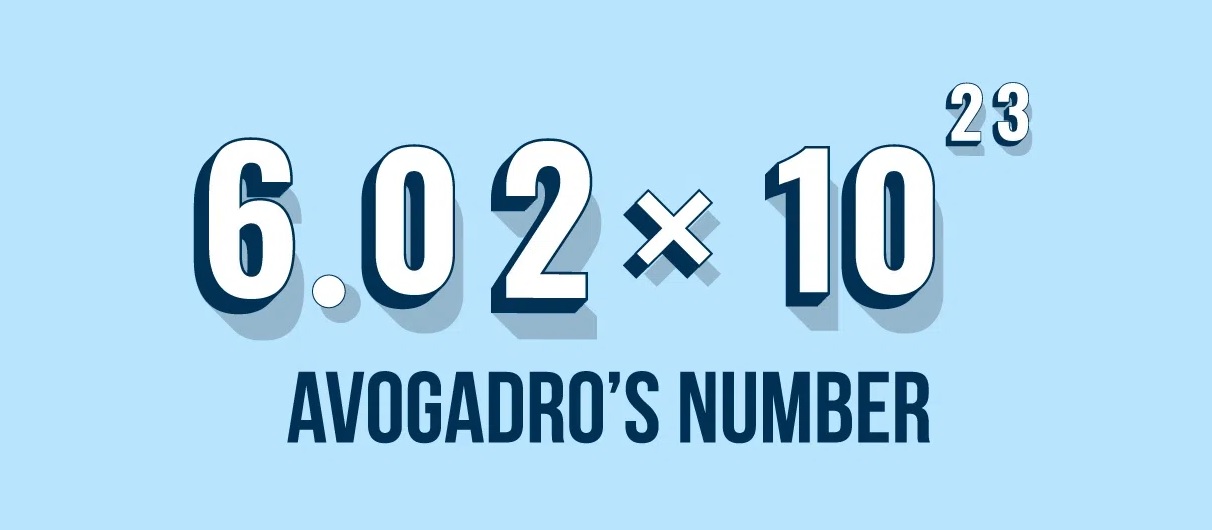
Avogadro’s number is a fundamental constant in chemistry that plays a crucial role in understanding the quantities of substances on a microscopic level. Named after the Italian scientist Amedeo Avogadro, this number represents the number of atoms or molecules in one mole of a substance. While Avogadro’s number may seem like a simple concept, it holds many fascinating facts and applications that contribute to our understanding of the molecular world.
In this article, we will explore 19 intriguing facts about Avogadro’s number that will not only deepen your knowledge of chemistry but also shed light on its significance in various scientific fields. From its historical origins to its implications in calculations and experiments, we will delve into the curious aspects of Avogadro’s number and its profound impact on the world of chemistry.
Key Takeaways:
- Avogadro’s Number, named after Amedeo Avogadro, helps scientists connect the world of atoms and molecules with observable properties, enabling precise measurements and calculations in chemistry.
- With its massive value of approximately 6.022 x 10^23, Avogadro’s Number serves as a constant reminder of the unimaginable number of atoms and molecules present in even the tiniest samples of matter, emphasizing the sheer scale of the atomic and molecular realm.
Avogadro’s Number is a fundamental constant in chemistry.
Known as Avogadro’s constant or Avogadro’s constant volume, it is denoted by the symbol NA and is equal to approximately 6.022 x 10^This constant represents the number of atoms or molecules in one mole of a substance.
Avogadro’s Number is named after the Italian scientist Amedeo Avogadro.
Amedeo Avogadro is renowned for his contributions to the understanding of molecular theory. His groundbreaking hypothesis, known as Avogadro’s Law, states that equal volumes of gases at the same temperature and pressure contain an equal number of molecules.
Avogadro’s Number allows scientists to bridge the gap between macroscopic and microscopic properties.
By quantifying the relationship between the mass of a substance and the number of particles it contains, Avogadro’s Number enables scientists to connect the world of atoms and molecules with observable properties on a larger scale.
Avogadro’s Number is crucial for stoichiometric calculations.
In stoichiometry, Avogadro’s Number is used to determine the mole ratio between reactants and products in a chemical reaction. It allows for precise measurement and calculation of the quantities of substances involved in a reaction.
Avogadro’s Number plays a significant role in the concept of molar mass.
The molar mass of a substance is defined as the mass of one mole of that substance. Avogadro’s Number is used to relate the mass of a sample to the number of moles it contains, allowing for accurate determination of molar mass.
Avogadro’s Number helps define the concept of the mole.
The mole is a fundamental unit in chemistry used to measure an amount of substance. Avogadro’s Number precisely quantifies the number of entities (atoms, molecules, ions) present in one mole of a substance.
Avogadro’s Number is a cornerstone of the scientific understanding of matter.
It provides a universal constant that allows scientists to perform calculations, analyze data, and make predictions in various fields of chemistry and physics.
Avogadro’s Number has practical applications in various industries.
From pharmaceuticals to materials science, Avogadro’s Number is instrumental in drug development, nanotechnology, and the study of polymers, among other applications.
Avogadro’s Number has a direct connection to the concept of the mole fraction.
The mole fraction is a way of expressing the concentration of a component in a mixture. Avogadro’s Number is used to determine the mole fraction of a particular substance within a solution.
Avogadro’s Number is utilized in the Avogadro’s law equation.
The equation PV = nRT, which is derived from Avogadro’s Law, involves Avogadro’s Number (NA) and is used to calculate the volume, pressure, amount, and temperature of an ideal gas.
Avogadro’s Number paves the way for the understanding of molecular weights.
The concept of molecular weight is closely connected to Avogadro’s Number. It allows for the calculation of molecular weights and the understanding of the relative masses of different particles.
Avogadro’s Number is related to the concept of the Avogadro constant volume.
The Avogadro constant volume is the number of particles in one mole of an ideal gas at standard temperature and pressure. It is equal to Avogadro’s Number and has significant implications in gas laws.
Avogadro’s Number enables scientists to determine the atomic and molecular masses of elements and compounds.
By utilizing Avogadro’s Number in conjunction with experimental data, chemists can accurately determine the atomic and molecular masses of substances, providing essential information for further analysis and research.
Avogadro’s Number helps scientists in understanding the concept of molar volume.
The molar volume is the volume occupied by one mole of a substance at a specific temperature and pressure. Avogadro’s Number aids in the determination and calculation of this important property.
Avogadro’s Number is an integral part of the ideal gas law equation.
The ideal gas law equation, PV = nRT, incorporates Avogadro’s Number, allowing scientists to describe the behavior of gases and make accurate predictions regarding their properties and interactions.
Avogadro’s Number provides a connection between the atomic scale and macroscopic observations.
By establishing a relationship between the number of particles in a sample and its observable properties, Avogadro’s Number allows scientists to bridge the gap between the molecular world and the world we perceive.
Avogadro’s Number is a fundamental concept in the field of physical chemistry.
Understanding and applying Avogadro’s Number is essential for comprehending various topics such as molecular kinetics, thermodynamics, and spectroscopy.
Avogadro’s Number is used to calculate the number of formula units in a substance.
By utilizing Avogadro’s Number, chemists can determine the number of formula units in a given amount of a compound, facilitating accurate measurements and calculations in chemical reactions.
Avogadro’s Number is a constant reminder of the vastness of the microscopic world.
With its massive value of approximately 6.022 x 10^23, Avogadro’s Number serves as a reminder of the unimaginable number of atoms and molecules present in even the tiniest samples of matter, emphasizing the sheer scale of the atomic and molecular realm.
In conclusion, Avogadro’s Number, with its profound implications in chemistry and its role in connecting the microscopic world of atoms and molecules with the macroscopic world we observe, is undoubtedly one of the most fascinating constants in scientific history. Its significance in stoichiometry, molar mass calculations, gas laws, and many other applications makes it a cornerstone of modern chemistry. Understanding Avogadro’s Number allows scientists to delve deeper into the complexities of matter and unlock new insights into the workings of the universe.
Conclusion
In conclusion, Avogadro’s number is a fundamental constant in chemistry that plays a crucial role in the study of matter at the atomic and molecular level. It is named after the Italian scientist Amedeo Avogadro, who proposed the concept of the mole and provided a way to link the macroscopic world to the microscopic world of atoms and molecules.
Avogadro’s number, approximately 6.022 x 10^23, represents the number of atoms or molecules in one mole of a substance. This constant allows scientists to make accurate measurements, calculate chemical formulas, and understand the behavior of matter in various chemical reactions.
Understanding the significance of Avogadro’s number not only helps in practical applications such as calculating concentrations and quantities in chemical reactions but also in comprehending the vastness and complexity of the microscopic world.
In summary, Avogadro’s number is a fascinating concept that forms the foundation of modern chemistry, bridging the gap between the macroscopic and microscopic realms.
FAQs
Q: What is Avogadro’s number?
Avogadro’s number is a fundamental constant in chemistry that represents the number of atoms or molecules in one mole of a substance. It is approximately 6.022 x 10^23.
Q: Who discovered Avogadro’s number?
Avogadro’s number is named after the Italian scientist Amedeo Avogadro, who proposed the concept of the mole and provided a way to link the macroscopic world to the microscopic world of atoms and molecules.
Q: What is the significance of Avogadro’s number?
Avogadro’s number is essential in chemistry as it allows scientists to make accurate measurements, calculate chemical formulas, and understand the behavior of matter in chemical reactions. It provides a fundamental connection between the atomic and macroscopic scale of matter.
Q: How is Avogadro’s number used in chemistry?
Avogadro’s number is used to calculate the number of particles (atoms or molecules) in a given amount of substance, determine molar mass, and convert between mass and moles in chemical calculations. It is also indispensable in understanding stoichiometry and balancing chemical equations.
Q: Is Avogadro’s number a constant?
Yes, Avogadro’s number is a constant that remains the same for all substances and under all conditions. It is a fundamental constant in nature and is universally applicable in chemistry.
Avogadro's number, a cornerstone of chemistry, continues to fascinate scientists and students alike. Dive deeper into the world of chemistry by exploring our collection of captivating chemistry facts. Uncover the mysteries surrounding the mole, a fundamental unit in chemistry, through our article on 18 intriguing mole facts. For those interested in chemical reactions and calculations, our piece on 11 enigmatic stoichiometry facts is a must-read. Embark on a journey of discovery and expand your knowledge of the fascinating world of chemistry with these engaging articles.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
