When it comes to understanding the intricate world of chemistry, the concept of bond angle plays a crucial role. Bond angle refers to the angle formed between two adjacent chemical bonds in a molecule. While it may seem like a simple concept, bond angles have extraordinary significance in determining the shape, polarity, and reactivity of molecules.
In this article, we will dive into the fascinating world of bond angles and explore 19 extraordinary facts that will deepen your understanding of this fundamental chemical concept. From the basics of bond angle measurement to the impact of bond angles on molecular behavior, this article aims to shed light on the importance and complexity of this topic.
So, grab your lab coat, put on your safety goggles, and prepare to be amazed by the intriguing world of bond angles!
Key Takeaways:
- Understanding bond angles helps predict the shape and properties of molecules, influencing everything from molecular polarity to hybridization and symmetry.
- Variances in bond angles can impact the strength of chemical bonds, molecular vibrations, and overall molecular symmetry, shaping the world of chemistry.
Bond angle dictates molecular geometry.
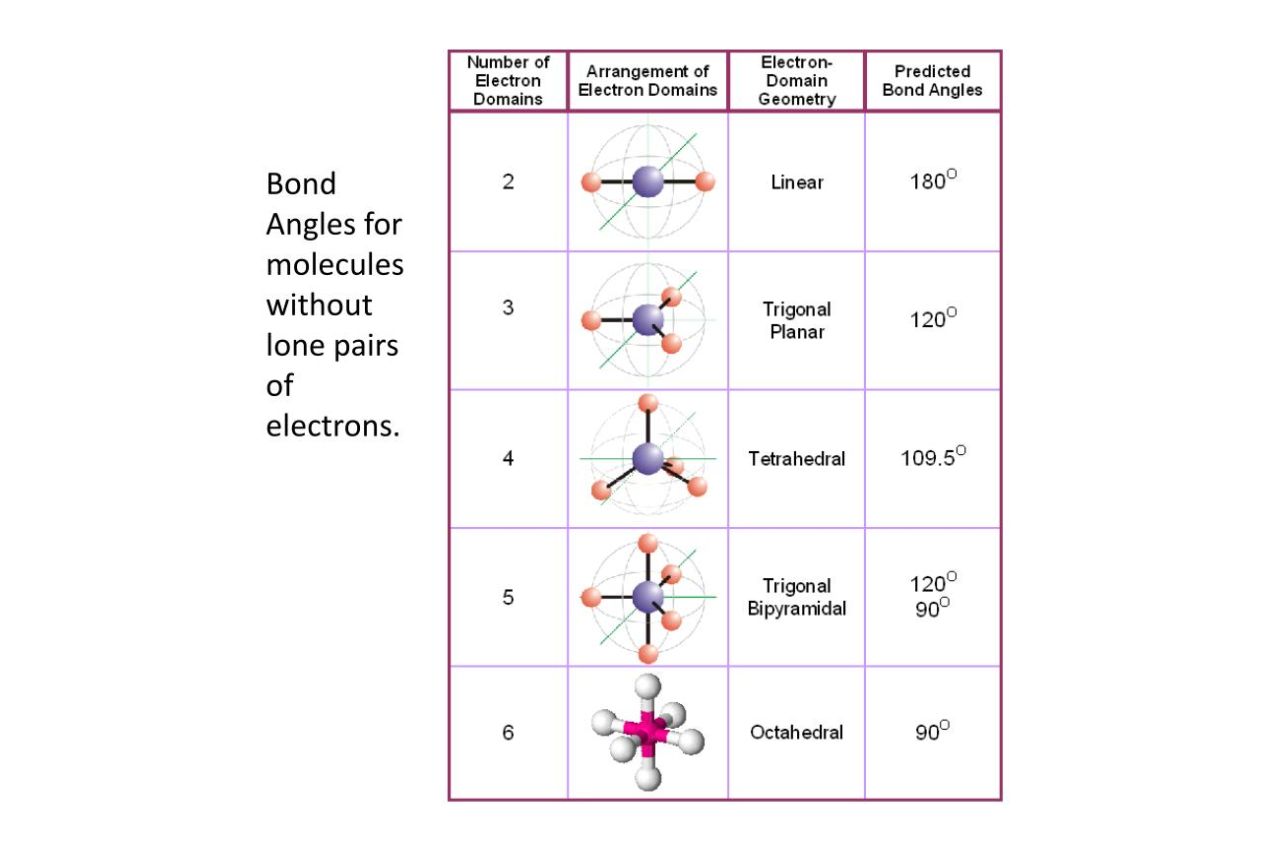
The bond angle influences the three-dimensional arrangement of atoms in a molecule. It determines whether a molecule is linear, trigonal planar, tetrahedral, or other complex shapes.
It contributes to bond strength.
The bond angle affects the overlap of atomic orbitals, influencing the strength and stability of chemical bonds between atoms.
Bond angles can vary.
Bond angles can range from 0° to 180°, depending on the atoms and the type of bonds involved. They can be acute, obtuse, or right angles.
Linear molecules have a bond angle of 180°.
In linear molecules, the bond angle between two bonds originating from the same atom is always 180°. Examples include carbon dioxide (CO2) and acetylene (C2H2).
The bond angle in a trigonal planar molecule is 120°.
Molecules with a trigonal planar geometry, like BF3 (boron trifluoride), have bond angles of 120°.
Tetrahedral molecules have a bond angle of 109.5°.
Tetrahedral-shaped molecules, such as methane (CH4) and carbon tetrachloride (CCl4), have bond angles of approximately 109.5°.
The bond angle affects molecular polarity.
Differences in bond angles can lead to variations in molecular polarity, influencing intermolecular forces and solubility properties.
Bond angles impact molecular vibrations.
The bond angle affects the vibrational frequencies and modes of a molecule, contributing to its infrared and Raman spectra.
Bond angle determines hybridization.
The bond angle can help predict the type of hybrid orbitals involved in bonding, such as sp, sp2, or sp3 hybridization.
Water (H2O) has a bond angle of 104.5°.
The bond angle in water, responsible for its bent molecular shape, is approximately 104.5°.
The bond angle in ammonia (NH3) is 107.3°.
Ammonia molecules exhibit a pyramidal shape with a bond angle of 107.3°.
Bond angles impact molecular dipoles.
Variances in bond angles affect the magnitude and direction of molecular dipoles, influencing overall molecular polarity.
Bond angles are affected by lone pairs.
Lone pairs of electrons around central atoms can distort bond angles, creating a bent or V-shaped molecular geometry.
The bond angle in a tetrahedral molecule decreases with lone pairs.
For molecules like ammonia (NH3) or water (H2O) with lone pairs, the bond angle decreases due to electron repulsion between lone pairs and bonding pairs.
Bond angles play a role in hybridization theory.
Hybridization theory explains the observed bond angles by combining atomic orbitals to form hybrid orbitals with specific geometries.
The bond angle in a square planar molecule is 90°.
In square planar molecules like XeF4 (xenon tetrafluoride), the bond angle is 90°.
Bond angles influence steric hindrance.
Large bond angles can result in increased steric hindrance, making certain molecular conformations less favorable.
Bond angles affect molecular symmetry.
The symmetry of a molecule is determined by its bond angles, which can be used to classify molecules into different point groups.
Bond angles can be measured using spectroscopy.
Techniques like X-ray crystallography and spectroscopy allow scientists to determine bond angles experimentally, providing crucial structural information about molecules.
These 19 extraordinary facts about bond angle highlight its significance in shaping the world of chemistry. Whether it’s determining molecular geometry, impacting bond strength, or influencing molecular properties, the bond angle plays a fundamental role in our understanding of chemical structures.
Conclusion
In conclusion, bond angle is a fascinating concept in chemistry that plays a crucial role in determining the shape and properties of molecules. Understanding bond angles helps scientists predict molecular behavior, study reactivity, and design new compounds.
Throughout this article, we have explored 19 extraordinary facts about bond angles, including their definition, types, influencing factors, and significance in various chemical phenomena. From the unique properties of water to the complexity of carbon-based compounds, bond angles prove to be a fundamental aspect of chemical bonding.
By delving into the intricate world of bond angles, we gain a deeper appreciation for the elegance and complexity of the molecular world. The study of bond angles continues to be a thriving field, with researchers unraveling new insights that contribute to advancements in materials science, drug development, and environmental research.
In summary, bond angle is an essential concept in chemistry that shapes our understanding and application of molecules. Exploring the intricacies of bond angles leads to remarkable discoveries and enables us to unlock endless possibilities in the realm of chemistry.
FAQs
What is a bond angle?
A bond angle is the angle between two adjacent chemical bonds in a molecule. It describes the spatial arrangement of atoms in a molecule and plays a critical role in determining its shape and reactivity.
How are bond angles determined?
Bond angles in a molecule are determined by the repulsion between electron pairs around a central atom. This repulsion, known as the VSEPR theory (Valence Shell Electron Pair Repulsion theory), governs the arrangement of atoms and lone pairs around a central atom, leading to specific bond angles.
What are some common bond angles?
Some common bond angles include 180 degrees in linear molecules (e.g., CO2), 120 degrees in trigonal planar molecules (e.g., BF3), and 109.5 degrees in tetrahedral molecules (e.g., CH4).
How do bond angles affect molecular properties?
Bond angles influence molecular properties such as bond strength, polarity, and biological activity. They dictate molecular shape, which, in turn, determines intermolecular forces, solubility, and chemical reactivity.
Why are bond angles important in organic chemistry?
In organic chemistry, bond angles play a crucial role in determining the shape and stability of organic molecules. They dictate the conformation of organic compounds, affecting their physical and chemical properties, reactivity, and biological activity.
Exploring bond angles is just the beginning of your chemistry journey. Dive deeper into the fascinating world of molecular shapes with our article on VSEPR theory, which explains how electron pairs influence geometry. For more captivating chemistry facts, check out our comprehensive collection that covers a wide range of topics. Don't miss our article on molecular geometry, where you'll discover 12 surprising facts about the spatial arrangement of atoms in molecules.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
