When it comes to the intricate world of chemistry, one of the most fascinating topics is the tertiary structure of molecules. The tertiary structure refers to the three-dimensional arrangement of atoms in a molecule, which determines its unique shape and function. This aspect of chemistry plays a crucial role in everything from drug design to understanding how enzymes work.
In this article, we will delve into the realm of tertiary structure and uncover 17 surprising facts that will amaze and captivate your curiosity. Get ready to explore the complex world of chemical structures and discover the hidden secrets that lie within. From the importance of disulfide bonds to the influence of temperature, these facts will give you a deeper understanding of the fascinating world of tertiary structure.
Key Takeaways:
- Tertiary structure refers to the 3D arrangement of atoms in proteins, crucial for their function and stability. Small changes can impact function, and studying this structure is vital for developing new treatments.
- Proteins can have different folding patterns, which affect their function. Understanding these structures helps scientists design targeted interventions for diseases like Alzheimer’s and Parkinson’s.
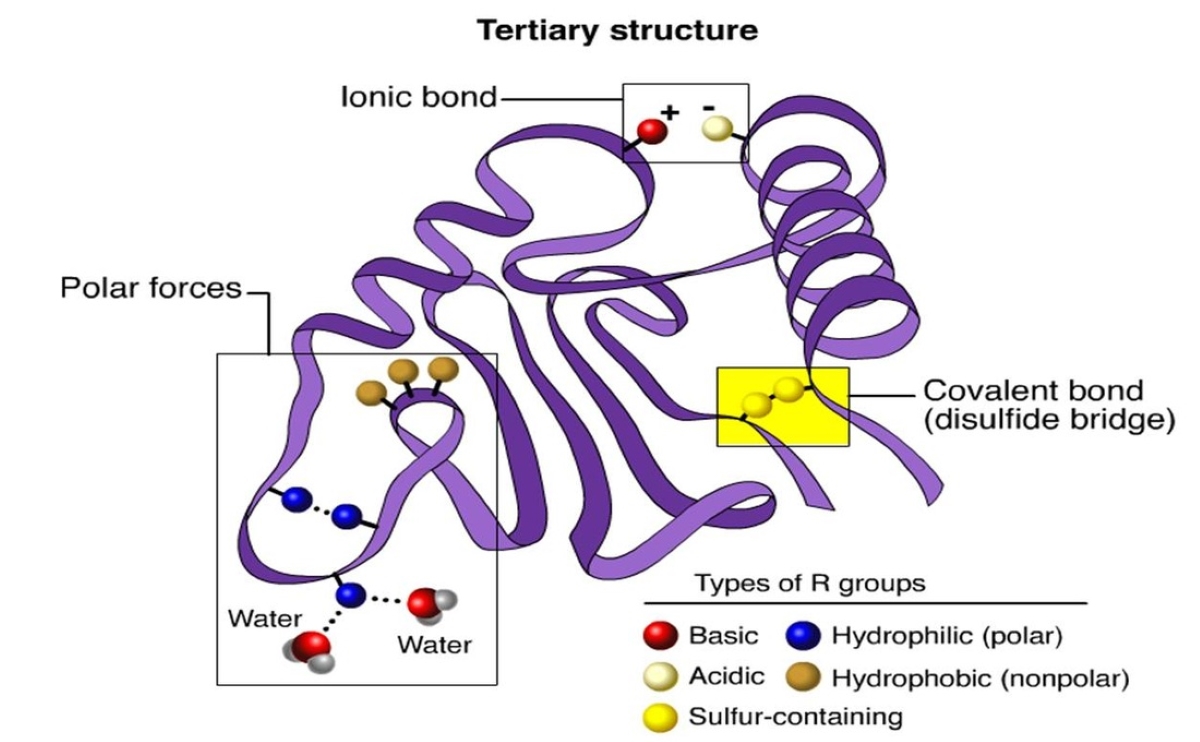
Tertiary structure refers to the three-dimensional arrangement of atoms in a protein.
The tertiary structure plays a crucial role in determining the protein’s function, stability, and interactions with other molecules.
Proteins can have different types of tertiary structures.
These include globular proteins, fibrous proteins, and membrane proteins. Each type has its own unique folding pattern and function.
The folding of proteins into their tertiary structure is driven by a combination of hydrophobic interactions, electrostatic interactions, and hydrogen bonding.
These forces help the protein to adopt its compact, folded shape.
Tertiary structure is essential for protein function.
The specific arrangement of amino acids in the tertiary structure allows proteins to perform their specific biochemical tasks, such as enzyme catalysis, signal transduction, and molecular recognition.
Small changes in the tertiary structure can have a significant impact on protein function.
A single amino acid substitution or modification can disrupt the folding and stability of the protein, leading to functional changes or even diseases.
The stability of the tertiary structure is influenced by various factors, such as pH, temperature, and the presence of denaturing agents.
Extreme conditions can cause proteins to denature and lose their functional tertiary structure.
Tertiary structure can be determined experimentally using techniques such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy.
These methods provide detailed insights into the arrangement of atoms within the protein.
Folding of proteins into their native tertiary structure is a complex and highly regulated process.
Chaperone proteins assist in the folding process and help prevent misfolding and aggregation of newly synthesized proteins.
Some proteins can adopt multiple conformations in their tertiary structure.
This conformational flexibility allows the protein to switch between different functional states and facilitates their interaction with ligands and other molecules.
Protein misfolding and aggregation are associated with various diseases.
Conditions such as Alzheimer’s, Parkinson’s, and prion diseases are characterized by the accumulation of misfolded proteins in the brain.
Tertiary structure can be altered by post-translational modifications.
Phosphorylation, glycosylation, and acetylation are examples of modifications that can affect protein folding and function.
The tertiary structure of a protein can be predicted computationally using algorithms and prediction tools.
These methods analyze the amino acid sequence and predict the most likely folding pattern based on known protein structures.
Tertiary structure is influenced by the primary and secondary structures of proteins.
The sequence of amino acids in the primary structure determines the folding pathway and the formation of secondary structural elements, which ultimately contribute to the overall tertiary structure.
Disulfide bonds play a crucial role in stabilizing the tertiary structure of some proteins.
These covalent bonds form between cysteine residues and help maintain the protein’s folded conformation.
The tertiary structure of proteins can be disrupted by chemical denaturants, such as urea and guanidine hydrochloride.
These agents destabilize the protein’s folding and unfold the structure, leading to loss of function.
Tertiary structure is not only limited to proteins.
RNA molecules can also adopt complex three-dimensional structures, known as tertiary structures, which are essential for their biological functions.
The study of tertiary structure is crucial for understanding protein structure-function relationships and designing new drugs and therapies.
Insights into the folding and stability of tertiary structures can help in the development of targeted interventions and treatments for various diseases.
Conclusion
In conclusion, the tertiary structure of proteins is a fascinating field that continues to unveil new surprises and insights into the complex world of molecules. Understanding the intricate folding and interactions that occur in the tertiary structure is crucial for comprehending the functionality and behavior of proteins in various biological processes. From the importance of disulfide bonds to the role of chaperone proteins, we now know that the tertiary structure plays a vital role in stabilizing and determining protein function. Additionally, the discovery of intrinsically disordered regions has revolutionized our understanding of protein structure and dynamics.
By unraveling these surprising facts about tertiary structure, scientists are able to gain a deeper understanding of the intricate world of proteins and their essential role in biological systems. As research progresses and new technologies emerge, we can expect to uncover even more intriguing and unexpected findings in the field of tertiary structure.
FAQs
Q: What is tertiary structure?
A: Tertiary structure refers to the three-dimensional arrangement and folding of a protein molecule. It is determined by the interactions between various amino acid residues, including hydrogen bonds, hydrophobic interactions, disulfide bonds, and electrostatic interactions.
Q: Why is tertiary structure important?
A: Tertiary structure is vital for protein functionality and stability. It determines the overall shape and surface properties of a protein, which in turn dictate its biological function and interactions with other molecules.
Q: How is tertiary structure different from primary and secondary structure?
A: Primary structure refers to the linear sequence of amino acids in a protein, whereas secondary structure refers to local arrangements such as alpha helices and beta sheets. Tertiary structure, on the other hand, describes the overall three-dimensional shape of the protein resulting from folding and interactions between different regions.
Q: What are intrinsically disordered regions?
A: Intrinsically disordered regions are segments within a protein that lack a well-defined structure. These regions play important roles in protein function and can undergo disorder-to-order transitions upon interacting with other molecules.
Q: How are disulfide bonds important in tertiary structure?
A: Disulfide bonds, formed between two cysteine residues, play a critical role in stabilizing the tertiary structure of proteins. They help to maintain the correct folding and stability of the protein by forming covalent bonds between specific regions.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
