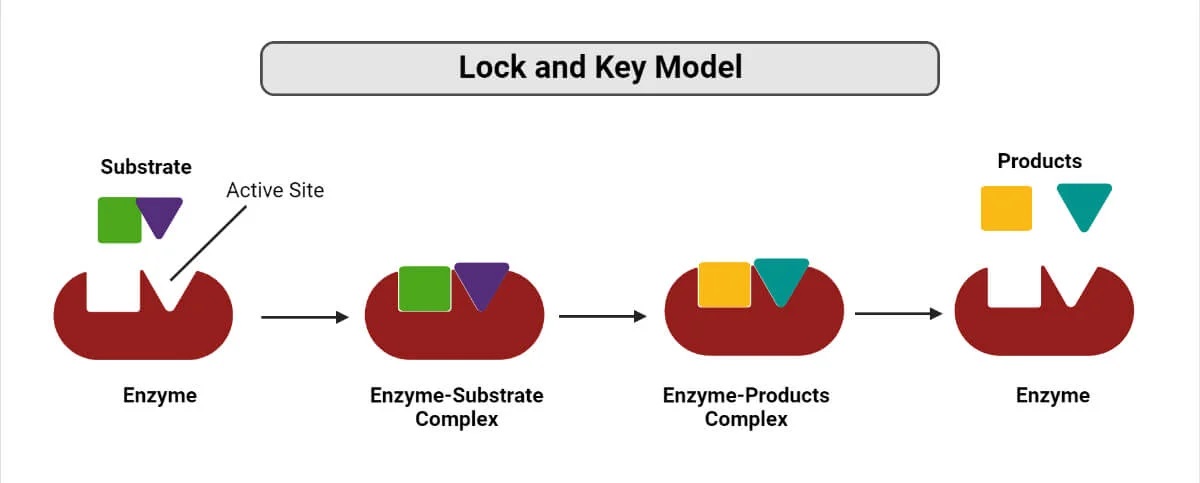
The lock-and-key model is a fundamental concept in chemistry that helps us understand how molecules interact and react with each other. This model, proposed by Emil Fischer in 1894, suggests that enzymes and substrates have complementary shapes that fit together like a lock and key, allowing for specific chemical reactions to take place.
In this article, we will explore 18 unbelievable facts about the lock-and-key model that will not only deepen our understanding of the concept but also showcase the remarkable intricacies of molecular interactions. From the amazing specificity of enzyme-substrate interactions to the role of molecular flexibility in catalysis, these facts will leave you in awe of the complexity and elegance of chemistry.
Key Takeaways:
- Enzymes and substrates interact like a lock and key, allowing enzymes to selectively bind to specific substrates for efficient biological reactions, just like a key fitting into a lock.
- The lock-and-key model not only revolutionized our understanding of enzyme-substrate interactions but also paved the way for drug design and new discoveries in biochemistry.
The Origin of the Lock-and-Key Model
The lock-and-key model, also known as the key-lock theory, was first proposed by Emil Fischer in He suggested that enzymes and substrates interact like a lock and key, where the active site of the enzyme acts as a lock and the substrate fits into it like a key. This model revolutionized our understanding of how enzymes catalyze biochemical reactions.
Enzymes Can Be Highly Specific
One of the remarkable aspects of the lock-and-key model is that enzymes exhibit high specificity towards their substrates. Just like a key that can only fit into a specific lock, enzymes can selectively bind to their specific substrates, leading to efficient catalysis of biological reactions.
Induced Fit Theory Modified the Lock-and-Key Model
The lock-and-key model was further refined by Daniel Koshland in 1958 through the introduction of the induced fit theory. According to this theory, the active site of the enzyme can slightly change its shape upon substrate binding, allowing for a better fit and optimal catalytic activity.
Enzymes Are Not Destroyed During Reactions
Contrary to the lock-and-key model’s implication that the enzyme and substrate are permanently bound, enzymes remain unchanged after catalyzing a reaction. They can be reused multiple times to catalyze the same reaction or other reactions, making them highly efficient catalysts in biological systems.
Lock-and-Key Model Applies to Receptor-Ligand Interactions
While initially applied to enzymes and substrates, the lock-and-key model is also applicable to receptor-ligand interactions. Receptors in the body, such as those involved in signal transduction or neurotransmission, can selectively bind to their ligands, triggering specific cellular responses.
Enzyme-Substrate Complex Stabilization
The lock-and-key model explains the stabilization of the enzyme-substrate complex. The binding between the enzyme and substrate is governed by various intermolecular forces, such as hydrogen bonding, electrostatic interactions, and hydrophobic interactions, ensuring a stable complex for efficient catalysis.
Substrate Specificity Determines Enzyme Function
Each enzyme has a specific substrate or group of substrates it can bind to and catalyze a reaction. The lock-and-key model helps us understand how the unique shape and chemical properties of an enzyme’s active site dictate its substrate specificity and ultimately its biological function.
Competitive Inhibition Disrupts the Lock-and-Key Interaction
Competitive inhibitors can disrupt the lock-and-key interaction by binding to the active site of the enzyme, preventing the substrate from binding. This interference leads to a decrease in enzymatic activity, highlighting the delicate balance in the lock-and-key interaction.
Lock-and-Key Model Simplifies Complex Biochemical Processes
The lock-and-key model provides a simplified representation of enzyme-substrate interactions. By conceptualizing the relationship between enzymes and substrates as a lock and key, scientists can better understand and study the complexity of biological processes and design strategies for drug development.
Temperature and pH Affect the Lock-and-Key Interaction
The lock-and-key interaction can be influenced by environmental factors such as temperature and pH. Changes in these conditions can alter the shape and charge distribution of the enzyme’s active site, affecting the binding affinity and catalytic efficiency.
Lock-and-Key Model Enables Enzyme Regulation
The lock-and-key model helps explain how enzymes can be regulated in living organisms. Modulating factors, such as inhibitors or activators, can interact with the enzyme to alter its conformation, thereby modifying its affinity for the substrate and regulating enzymatic activity.
Lock-and-Key Model Guides Drug Design
The lock-and-key model has been instrumental in guiding drug design. By understanding the specific interactions between an enzyme’s active site and its substrate, scientists can design inhibitors or drugs that selectively bind to the enzyme, modulating its activity for therapeutic purposes.
Proteins Display Lock-and-Key Interactions
In addition to enzymes, lock-and-key interactions are also observed in protein-protein interactions. Proteins can bind to each other in a specific and selective manner, facilitating the formation of complexes critical for various cellular processes.
The Lock-and-Key Model Illustrates Enzyme Specificity
The remarkable specificity of enzymes is central to their function, and the lock-and-key model effectively demonstrates this concept. Just as a specific key can open a particular lock, each enzyme is uniquely suited to interact with its specific substrate and carry out its catalytic function.
Lock-and-Key Model Supports Enzyme Kinetics
The lock-and-key model provides the foundation for understanding enzyme kinetics. By studying the rate at which enzymes catalyze reactions under different conditions, scientists can unravel the intricacies of the lock-and-key interaction and gain insights into enzymatic behavior.
Enzyme Flexibility Influences Lock-and-Key Interactions
While the lock-and-key model suggests a rigid interaction between enzymes and substrates, enzymes actually exhibit a degree of flexibility. This flexibility allows the enzyme to accommodate different substrates and adapt its active site for efficient catalysis.
Lock-and-Key Model Applies to Non-Enzymatic Interactions
Beyond enzymes, the lock-and-key model has found applications in understanding non-enzymatic interactions, such as antibody-antigen recognition or DNA-protein binding. The concept of selective binding and complementary molecular shapes is fundamental to various biological processes.
Lock-and-Key Model Paves the Way for New Discoveries
The lock-and-key model continues to be a cornerstone in the field of biochemistry and has laid the foundation for further research and discoveries. By unraveling the intricacies of enzyme-substrate interactions, scientists have gained insights into the complexity of biological systems and continue to explore new frontiers in enzymology.
Conclusion
The lock-and-key model is truly fascinating in the field of chemistry. Understanding how molecules interact and bind to each other is essential in studying various chemical reactions and processes. The concept of the lock-and-key model provides us with a visual representation of these interactions, helping us to better comprehend the intricate world of chemistry.
By delving into the 18 unbelievable facts about the lock-and-key model, we have gained insight into the importance of molecular recognition, enzyme specificity, and the role of shape and complementarity in chemical reactions. We have also explored how this model has influenced various areas of chemistry, including drug design, protein structure analysis, and biochemistry.
Overall, the lock-and-key model serves as a fundamental principle that underpins many aspects of chemistry. By understanding its intricacies, we can unlock new possibilities in the field and continue to make groundbreaking discoveries.
FAQs
1. What is the lock-and-key model in chemistry?
The lock-and-key model is a concept that explains how molecules interact with one another. It suggests that molecules are like keys that fit into specific lock-like receptors, forming a stable complex.
2. How does the lock-and-key model contribute to drug design?
The lock-and-key model is crucial in drug design as it helps scientists understand how drugs can selectively bind to specific receptors in the body. By designing molecules that can fit into these receptors, scientists can develop drugs that have desired therapeutic effects.
3. What is the significance of shape and complementarity in the lock-and-key model?
In the lock-and-key model, the shape of the receptor and the molecule must be complementary for binding to occur. This ensures specificity and proper functionality of the molecular complex.
4. How does the lock-and-key model explain enzyme specificity?
The lock-and-key model suggests that enzymes have specific active sites that can only accommodate molecules with the correct shape and properties. This specificity allows enzymes to catalyze specific reactions and regulate biochemical processes.
5. How has the lock-and-key model influenced protein structure analysis?
The lock-and-key model has provided valuable insights into understanding the three-dimensional structure of proteins. By understanding how molecules interact and bind, scientists can determine the folding and function of proteins.
6. Can the lock-and-key model be applied to other areas of science?
While the lock-and-key model originates from chemistry, its principles of molecular recognition and binding can be applied to various fields such as biochemistry, pharmacology, and materials science.
Unraveling the secrets of the lock-and-key model is just the beginning of your journey into the fascinating world of biochemistry. Protein structure holds the key to understanding how these complex molecules function and interact within living organisms. Enzyme kinetics shed light on the incredible speed and efficiency of biochemical reactions, while enzyme regulation ensures that these powerful catalysts are kept in check. Dive deeper into these captivating topics and expand your knowledge of the intricate workings of life at the molecular level.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
