Amphiprotic solvents are a fascinating category of chemicals that possess the unique ability to behave as both acids and bases. This dual nature makes them incredibly versatile and essential in various chemical processes and reactions. From a chemistry standpoint, studying amphiprotic solvents provides insights into their dynamic behavior and how they interact with different substances.
In this article, we will explore 17 surprising facts about amphiprotic solvents that will deepen your understanding of their properties and applications. From their role in acid-base reactions to their significance in organic synthesis, these solvents play a vital role in the field of chemistry. So, let’s dive into the intriguing world of amphiprotic solvents and uncover some fascinating insights!
Key Takeaways:
- Amphiprotic solvents can act as both acids and bases, dissolve a wide range of substances, and have diverse applications in industries and chemical experiments.
- Water is the most well-known amphiprotic solvent, playing a vital role in biological and chemical reactions, and influencing pH levels in solutions.
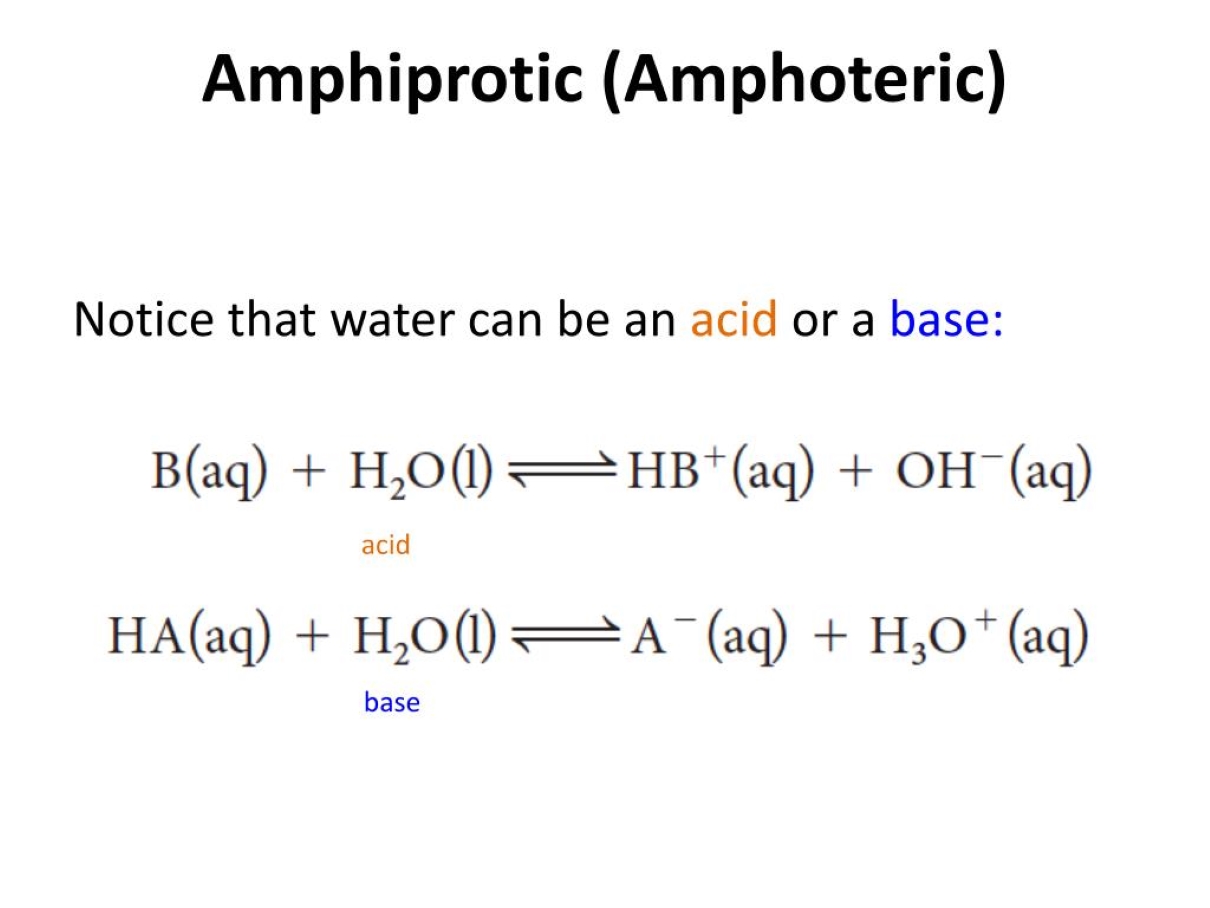
Amphiprotic solvents can act as both acids and bases.
Amphiprotic solvents possess the unique ability to donate and accept protons, allowing them to exhibit both acidic and basic properties. This versatility makes them invaluable in various chemical reactions and processes.
They can dissolve both polar and nonpolar substances.
One of the remarkable characteristics of amphiprotic solvents is their ability to dissolve a wide range of substances, regardless of their polarity. This versatility makes them essential in many industrial applications and chemical experiments.
Amphiprotic solvents have a wide range of applications.
From pharmaceuticals to organic synthesis, these solvents find use in diverse fields. They are commonly employed in drug formulations, electrochemical studies, and as reaction media in various chemical processes.
Water is the most well-known amphiprotic solvent.
Water, an essential component of life, is a prime example of an amphiprotic solvent. With its ability to both donate and accept protons, it plays a vital role in numerous biological and chemical reactions.
Amphiprotic solvents are crucial in acid-base titrations.
Due to their dual nature, these solvents are used extensively in acid-base titrations to determine the concentration of an acid or base in a solution. Their ability to accept or donate protons allows for precise measurement of the equivalence point.
They can act as good electrolytes.
Amphiprotic solvents, including certain species of alcohol and water, exhibit excellent electrical conductivity due to the presence of ions. This property is exploited in fuel cells, batteries, and other electrochemical devices.
Amphiprotic solvents have the property of autodissociation.
Autodissociation refers to the ability of these solvents to spontaneously undergo a chemical reaction with themselves, resulting in the formation of hydronium and hydroxide ions. This self-ionization is particularly evident in water.
Common examples of amphiprotic solvents include methanol and acetic acid.
Methanol and acetic acid are well-known amphiprotic solvents frequently used in laboratory settings. They find utility in various organic reactions, as well as in the production of pharmaceuticals and fine chemicals.
The amphiprotic nature of solvents can influence reaction rates.
The ability of amphiprotic solvents to donate and accept protons can significantly impact reaction rates. Depending on the specific reaction, these solvents can either accelerate or hinder the progression of chemical transformations.
Amphiprotic solvents are commonly used as solvents for metal ions.
The dual nature of these solvents makes them suitable for solubilizing and stabilizing metal ions. Their ability to coordinate with metal centers allows for the formation of stable complexes essential in various industrial applications.
They play a crucial role in acid-catalyzed reactions.
Amphiprotic solvents, due to their ability to act as both acids and bases, are often employed as reaction media in acid-catalyzed transformations. This property enables the activation of certain reactants and facilitates the desired chemical conversions.
The autoionization of water is an essential concept related to amphiprotic solvents.
The phenomenon of water’s autoionization, where it spontaneously dissociates into hydronium and hydroxide ions, is fundamental in understanding the amphiprotic nature of solvents. This property allows water to participate in a wide range of chemical reactions.
The amphiprotic nature of solvents affects pH levels.
Amphiprotic solvents, such as water, can influence the pH of a solution. Depending on the concentration of hydronium and hydroxide ions, these solvents can exhibit acidic, neutral, or basic properties, thus affecting the overall acidity or alkalinity of a system.
Amphiprotic solvents are commonly used in spectroscopy.
Due to their ability to dissolve a wide range of substances, amphiprotic solvents serve as excellent media for spectroscopic techniques. They allow for the accurate measurement and analysis of compounds through methods like UV-Visible spectrophotometry and nuclear magnetic resonance (NMR).
The solvent strength of amphiprotic solvents can vary.
Just like other solvents, the ability of amphiprotic solvents to dissolve solutes can vary widely depending on factors such as temperature, pressure, and the nature of the solute. These variations in solvent strength contribute to the versatility of these solvents.
Amphoteric substances can behave as amphiprotic solvents.
In addition to the commonly known amphiprotic solvents, certain amphoteric compounds can also act as solvents with both acidic and basic properties. These substances, such as certain metal oxides and hydroxides, exhibit versatile behavior in various chemical systems.
Amphiprotic solvents have implications in biological systems.
Given the significance of water as an amphiprotic solvent in biological systems, the behavior and properties of these solvents have implications for many biological processes. The ability of water to serve as a medium for biochemical reactions is essential for life as we know it.
Conclusion
Amphiprotic solvents are truly fascinating substances with unique properties that make them versatile in various chemical and industrial applications. From their ability to act as both acids and bases to their crucial role in maintaining pH balance, these solvents have a significant impact on chemical reactions and biological systems.Throughout this article, we have explored 17 surprising facts about amphiprotic solvents. We have learned how they can donate or accept protons, their role as buffer solutions, and their ability to promote various chemical reactions. We have also discovered some lesser-known facts, such as their applications in industries like pharmaceuticals, cosmetics, and environmental remediation.Understanding the properties of amphiprotic solvents is fundamental in many fields such as chemistry, biochemistry, and environmental science. By harnessing the unique attributes of these solvents, researchers and scientists can advance various technologies and improve processes across industries.In conclusion, the study of amphiprotic solvents is crucial for expanding our knowledge of chemical interactions and their impact on the world around us. By delving deeper into the fascinating world of amphiprotic solvents, we can unlock new possibilities and pave the way for exciting advancements in science and technology.
FAQs
Q: What is an amphiprotic solvent?
An amphiprotic solvent is a substance that can both donate and accept protons (H+ ions), depending on the circumstances. It acts as both an acid and a base due to its unique chemical properties.
Q: How does an amphiprotic solvent maintain pH balance?
Amphiprotic solvents can act as buffer solutions, which means they can resist changes in pH when small amounts of acid or base are added. They can donate or accept protons to maintain a stable pH environment.
Q: What are some common examples of amphiprotic solvents?
Water is the most well-known amphiprotic solvent. Other examples include methanol, acetic acid, and ammonia.
Q: What are the main applications of amphiprotic solvents?
Amphiprotic solvents find applications in various industries such as pharmaceuticals, cosmetics, and environmental remediation. They are also essential in chemical reactions, biochemical processes, and maintaining pH balance in biological systems.
Q: Can amphiprotic solvents be hazardous?
Like any chemical substance, amphiprotic solvents can be hazardous if not handled properly. It is crucial to follow proper safety protocols and guidelines when working with these solvents to minimize any potential risks.
Amphiprotic solvents' unique properties make them essential in various chemical applications. Dive deeper into the world of chemistry by exploring our collection of captivating chemistry facts. Uncover the secrets of molecular structure with our article on the fascinating facts about orbital hybridization. For those curious about the enigmatic realm of physical chemistry, our piece on its intriguing aspects is a must-read.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
