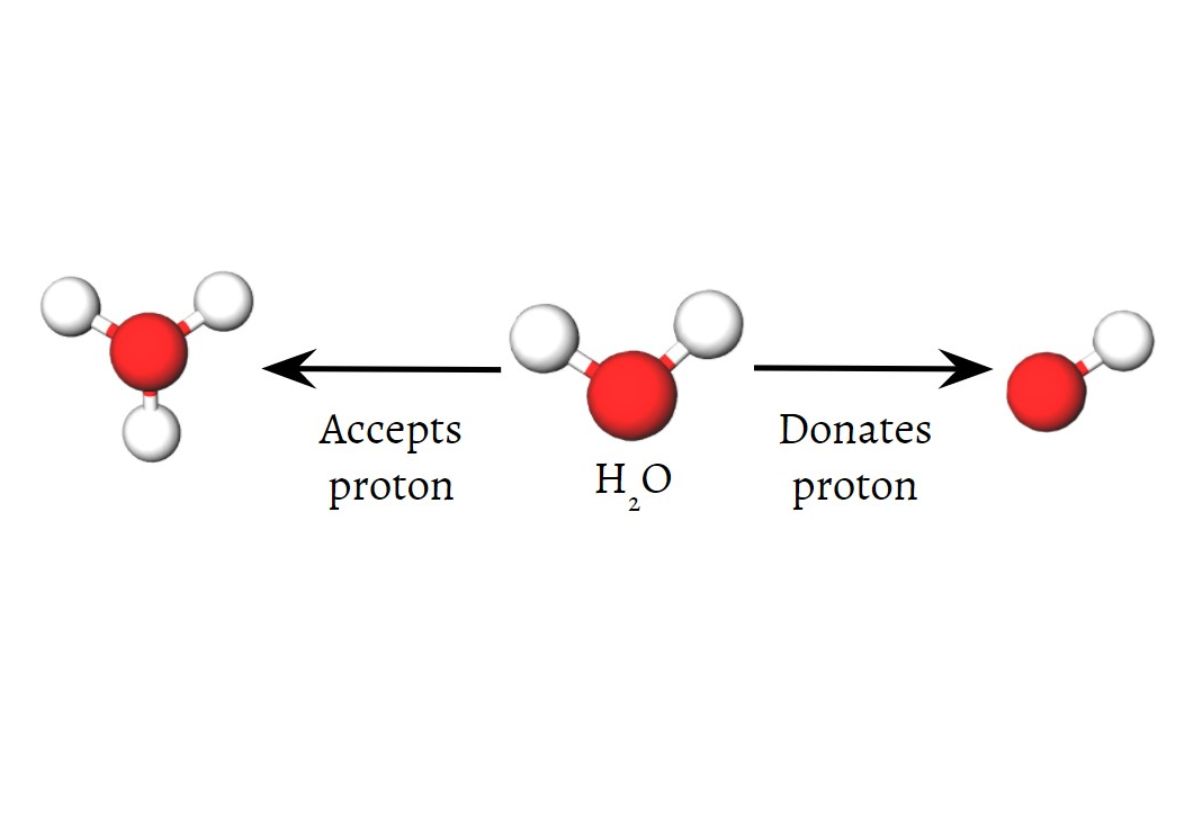
When it comes to the fascinating world of chemistry, there are countless topics to explore. One such topic that sparks curiosity is amphiprotic species. These unique chemical compounds have the remarkable ability to both accept and donate protons. This dual nature makes amphiprotic species incredibly versatile and significant in various chemical reactions and biological processes.
In this article, we will dive deep into the world of amphiprotic species and uncover 18 captivating facts that will leave you intrigued. From understanding the concept of amphiproticity to exploring real-life examples and applications, this article will shed light on the intriguing nature of these compounds. So, let’s uncover the secrets behind the fascinating world of amphiprotic species and discover why they are of paramount importance in the field of chemistry.
Key Takeaways:
- Amphiprotic species can act as both acids and bases, playing a crucial role in chemical reactions and maintaining balance in the world of chemistry.
- Understanding amphiprotic species is like unlocking a secret code in chemistry, revealing their dual nature and significant impact across scientific fields.
Amphiprotic species can act as both acid and base.
Amphiprotic species are fascinating in the world of chemistry because they possess the unique ability to act as both an acid and a base. They can donate or accept protons depending on the conditions they are in.
Water is the most well-known amphiprotic species.
Water, the essential component of life, is an amphiprotic species. It can donate a proton to behave as an acid or accept a proton to behave as a base.
Amphiprotic species have a dual nature.
Amphiprotic species demonstrate a dual nature, being able to exhibit acidic properties in a basic environment and basic properties in an acidic environment.
The ability of amphiprotic species is influenced by pH.
The behavior of amphiprotic species is highly dependent on the pH of the surrounding medium. They will exhibit more acidic properties under basic conditions and more basic properties under acidic conditions.
Amphiprotic species play a crucial role in acid-base reactions.
Amphiprotic species are key players in acid-base reactions, as they can either donate or accept protons to facilitate the balance of charges in the reaction.
Common examples of amphiprotic species include amino acids.
Amino acids, the building blocks of proteins, are prime examples of amphiprotic species. They have both an amino group (basic) and a carboxyl group (acidic) in their structure.
Some metal ions can also exhibit amphiprotic behavior.
Certain metal ions, such as aluminum and zinc, can display amphiprotic properties. They can act as Lewis acids by accepting electron pairs and as Lewis bases by donating electron pairs.
Amphoteric compounds are another name for amphiprotic species.
Amphiprotic species are sometimes referred to as amphoteric compounds. The term “amphoteric” emphasizes their dual nature in being able to act as an acid or base.
The concept of amphiprotic species was first proposed by Gilbert N. Lewis.
The notion of amphiprotic species was introduced by American chemist Gilbert N. Lewis, known for his contributions to the understanding of chemical bonding.
Amphiprotic behavior extends beyond individual molecules.
Not only can individual molecules exhibit amphiprotic behavior, but entire substances, such as hydroxides, oxides, and certain salts, can also demonstrate this property.
The pH at which an amphiprotic species is neutral is called its isoelectric point.
Each amphiprotic species has a unique isoelectric point, which is the pH at which it is electrically neutral.
Proteins rely on the amphiprotic properties of amino acids for proper functioning.
The amphiprotic nature of amino acids plays a vital role in the structure and function of proteins. It allows them to maintain the necessary balance of charges for their overall stability and activity.
The amphiprotic character of a species can be predicted using Lewis acid-base theory.
Lewis acid-base theory can provide insights into the amphiprotic behavior of a species by examining its ability to accept or donate electron pairs.
The presence of amphiprotic species can affect the pH of a solution.
If an amphiprotic species is present in a solution, it can contribute to the ionic equilibrium and potentially impact the pH of the solution.
The strength of the amphiprotic behavior varies among species.
Not all amphiprotic species exhibit the same level of amphiprotic behavior. Some may be more inclined to behave as an acid, while others may lean towards acting as a base.
Bromate ion (BrO3-) is an example of an amphiprotic species.
The bromate ion, BrO3-, is considered an amphiprotic species as it can either donate or accept protons depending on the conditions it encounters.
Amphiprotic properties are significant in understanding buffer systems.
Buffer systems rely on the amphiprotic behavior of certain species to maintain a stable pH and prevent drastic changes in acidity or alkalinity.
Understanding amphiprotic species is crucial in many fields, including biology and environmental science.
The knowledge of amphiprotic species is not only important in the field of chemistry but also finds applications in various scientific disciplines, such as biology, environmental science, and pharmaceuticals.
Conclusion
In conclusion, amphiprotic species are truly fascinating. They possess the ability to donate and accept protons, making them unique in the world of chemistry. Their versatility is evident in various applications, ranging from biological processes to chemical reactions. Understanding the properties and behavior of amphiprotic species is crucial for researchers and scientists in advancing our knowledge and finding new ways to utilize these compounds.Exploring the world of amphiprotic species opens up a whole new realm of possibilities, leading to exciting discoveries and advancements in science. Whether it is studying the role of amphiprotic species in acid-base reactions or discovering new ways to design molecules with amphiprotic properties, these compounds continue to captivate chemists around the globe.As we continue to delve deeper into the complexities of chemistry, the study of amphiprotic species will undoubtedly contribute to our understanding of the fundamental principles that govern the natural world. So, let us embrace the intrigue of amphiprotic species and continue to unravel the mysteries of these captivating compounds.
FAQs
Q: What does it mean for a species to be amphiprotic?
A: Amphiprotic species are capable of both donating and accepting protons (H+ ions) in a chemical reaction. They can act as acids, donating protons, or as bases, accepting protons, depending on the reaction conditions.
Q: How does the amphiprotic nature of a species affect its behavior in a solution?
A: The amphiprotic nature of a species allows it to maintain a balanced pH in a solution. It can act as an acid to neutralize excess hydroxide ions and as a base to neutralize excess hydrogen ions, helping to stabilize the pH of the solution.
Q: Can you provide examples of amphiprotic species?
A: Some examples of amphiprotic species include water (H2O), hydrogen carbonate ion (HCO3-), and amino acids such as glycine. These compounds can donate or accept protons depending on the reaction conditions.
Q: How are amphiprotic species involved in acid-base reactions?
A: In acid-base reactions, amphiprotic species can act as either an acid or a base. They can donate a proton to a base or accept a proton from an acid, facilitating the transfer of protons between species and the formation of new compounds.
Q: Are all substances amphiprotic?
A: No, not all substances are amphiprotic. Amphiprotic species possess specific molecular properties that allow them to donate and accept protons. Most substances are either exclusively acidic or basic and do not exhibit amphiprotic behavior.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
