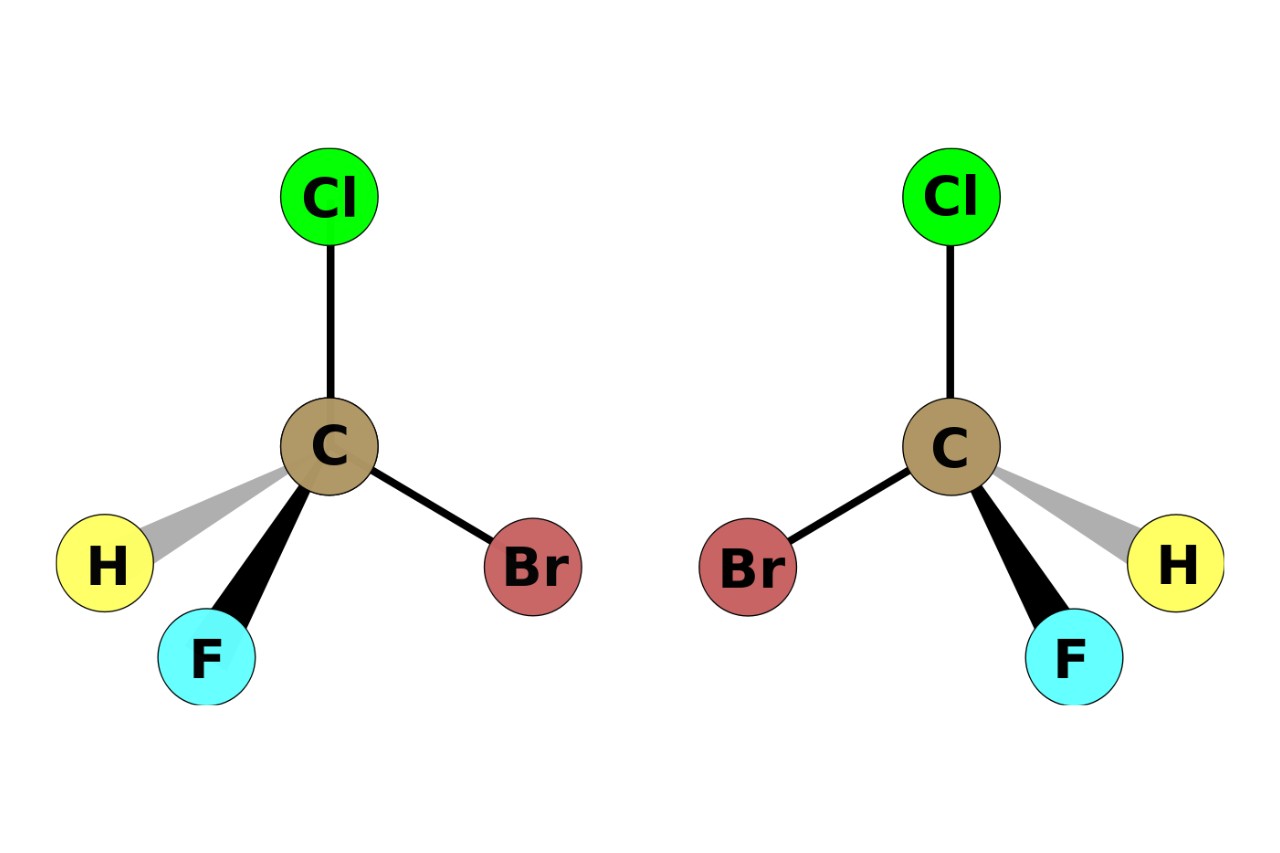
Optical isomerism is a fascinating phenomenon in the world of chemistry. It refers to the property of certain molecules to exist in two different forms that are each other’s mirror images but cannot be superimposed on one another. These mirror-image molecules are known as enantiomers, and they play a crucial role in many aspects of chemistry, biology, and pharmacology.
In this article, we will explore 16 unbelievable facts about optical isomerism that will not only surprise you but also deepen your understanding of this intriguing concept. From the discovery of enantiomers to their significance in drug design and how they affect our sense of taste and smell, get ready to delve into the remarkable world of optical isomerism.
Key Takeaways:
- Optical isomerism creates mirror-image molecules with different properties, impacting fields like pharmaceuticals and biology. Understanding it is crucial for scientists and leads to new discoveries.
- Enantiomers, the non-superimposable mirror images in optical isomerism, have identical physical properties but different biological and chemical activities. This phenomenon has significant implications in various industries.
Optical Isomerism Explained
Optical isomerism, also known as enantiomerism, is a fascinating phenomenon in chemistry. It occurs when molecules have a non-superimposable mirror image, resulting in different physical and chemical properties.
Chiral Structures
Optical isomerism arises from the presence of chiral structures in a molecule. A molecule is chiral if it does not possess an internal plane of symmetry.
Hand and Glove Analogy
A common way to visualize optical isomerism is through the hand and glove analogy. Just like a left and right hand cannot be superimposed, enantiomers cannot be superimposed onto their mirror images.
Biological Significance
Optical isomerism plays a crucial role in biology. Many biological molecules, such as amino acids and sugars, exist as enantiomers. The different properties of these enantiomers can have profound effects on biological processes.
Stereochemistry
Stereochemistry is the branch of chemistry that studies the spatial arrangements of atoms in molecules and the effects of these arrangements on chemical reactions.
Enantiomers vs. Diastereomers
Enantiomers are non-superimposable mirror images, while diastereomers are stereoisomers that are not mirror images of each other. Optical isomerism specifically refers to the existence of enantiomers.
Different Physical Properties
Enantiomers have identical physical properties, such as boiling points and melting points. However, they exhibit different optical activities, rotation of plane-polarized light, which is a distinguishing characteristic of optical isomerism.
Pharmaceutical Importance
Optical isomerism is of great significance in the pharmaceutical industry. Enantiomers often have different pharmacological activities, and one enantiomer may be more effective while the other can potentially cause adverse effects.
Thalidomide Tragedy
The thalidomide tragedy is one of the most notorious examples of the consequences of overlooking optical isomerism. The drug was marketed as a sedative in the 1950s but later discovered to cause severe birth defects.
Synthesis and Separation
Chemists use various methods to synthesize and separate enantiomers. Chiral catalysts, resolution techniques, and chromatography are commonly employed in the production and isolation of enantiopure compounds.
Mirror Image Properties
Enantiomers have the same chemical formula and connectivity of atoms. However, they can interact differently with other chiral molecules, showing distinct biological and chemical activities.
Molecular Symmetry
In order for a molecule to exhibit optical isomerism, it must lack both a plane of symmetry and an internal axis of rotation. These elements will result in the molecule being superimposable onto its mirror image.
Pharmacokinetics
Pharmacokinetics refers to how drugs are absorbed, distributed, metabolized, and excreted by the body. Enantiomers can be metabolized differently, leading to variations in drug efficacy and toxicity.
Ways to Represent Enantiomers
Enantiomers are often labeled as R and S or (+) and (-) based on their specific orientation in 3D space. These labels help distinguish between the two mirror-image forms.
Optical Purity
Optical purity is a measure of the extent to which a sample of a compound contains one enantiomer versus the other. It is commonly determined using polarimetry or chiral chromatography.
Everyday Examples
Optical isomerism can be observed in everyday objects. For instance, the scent of lemons is due to the presence of the enantiomer of limonene.
These 16 unbelievable facts about optical isomerism highlight its significance in chemistry and its impact on various disciplines, from pharmaceuticals to biology. Understanding the concept of optical isomerism is essential for scientists and researchers, as it offers insights into the behavior and properties of molecules. The study of optical isomerism continues to unravel new discoveries and application possibilities in diverse fields.
Conclusion
In conclusion, optical isomerism is a fascinating aspect of chemistry that involves the study of molecules with mirror-image symmetry. These compounds, known as enantiomers, exhibit unique properties and interactions due to their asymmetry. Understanding optical isomerism is crucial in fields such as pharmaceuticals, biochemistry, and material science.
Through this article, we have explored 16 unbelievable facts about optical isomerism. We have learned about the significance of chirality, the mechanisms of optical activity, and the ways in which scientists can identify and separate enantiomers.
As researchers continue to delve into the mysteries of optical isomerism, we can expect new discoveries and applications in various industries. By harnessing the power of enantiomers, scientists can develop more effective drugs, improve catalytic processes, and enhance the performance of materials.
Optical isomerism is an enduring topic of study, and its implications are vast. By delving deeper into the world of chiral molecules, we gain a greater understanding of the complex nature of chemistry and the wonders that lie within it.
FAQs
Q: What is optical isomerism?
A: Optical isomerism refers to the phenomenon exhibited by certain compounds that are non-superimposable mirror images of each other. These compounds are called enantiomers.
Q: Why is optical isomerism important?
A: Optical isomerism is important because enantiomers often have different biological activities and interactions. It plays a crucial role in drug design, as even a slight change in the arrangement of atoms can result in different therapeutic effects.
Q: How can enantiomers be distinguished?
A: Enantiomers can be distinguished using techniques such as polarimetry, which measures their ability to rotate plane-polarized light. Additionally, chiral chromatography and nuclear magnetic resonance (NMR) spectroscopy can be employed.
Q: What are some examples of optically active compounds?
A: Some examples of optically active compounds include certain sugars (like glucose and fructose), amino acids, and pharmaceutical drugs such as ibuprofen and penicillin.
Q: Can enantiomers be separated?
A: Yes, enantiomers can be separated through techniques such as crystallization, chromatography, and the use of chiral catalysts. These methods help isolate and purify individual enantiomers.
Optical isomerism's fascinating world extends beyond simple molecules. Coordination complexes display captivating instances of this phenomenon, with their unique structures and properties. Geometric isomerism, another intriguing aspect of stereochemistry, reveals how spatial arrangement profoundly impacts chemical behavior. Delving deeper into these topics promises to enrich your understanding of chemistry's marvels.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
