When it comes to understanding the interactions between different molecules, the concept of molecular polarity plays a pivotal role. The polarity of a molecule refers to the distribution of its electron density, which determines how it interacts with other molecules. In the realm of chemistry, molecular polarity is a fascinating subject that can shed light on a wide range of phenomena.
In this article, we will dive into the world of molecular polarity and explore ten extraordinary facts that highlight its importance and impact. From understanding the role of polarity in chemical reactions to its influence on physical properties, we will unravel the mysteries behind this concept. So, prepare to delve into the intriguing world of molecular polarity and discover the many ways it shapes the chemistry that surrounds us.
Key Takeaways:
- Molecular polarity affects how substances interact and behave in the world. It determines solubility, boiling points, and even the behavior of biological systems.
- Understanding molecular polarity helps scientists predict how substances will behave, design new materials, and unravel the complexities of biological systems.
Definition of Molecular Polarity
Molecular polarity refers to the uneven distribution of electron density within a molecule. It occurs when there is a significant difference in electronegativity between atoms in a covalent bond. This uneven distribution creates regions of partial positive and partial negative charges, resulting in a polar molecule.
Determining Molecular Polarity
The polarity of a molecule is determined by two factors: the polarity of its individual bonds and the molecular geometry. If a molecule has polar bonds, but its geometry is symmetrical, the polarities can cancel each other out, resulting in a nonpolar molecule. On the other hand, if the geometry is asymmetrical, the molecule will be polar.
Influence of Molecular Polarity on Solubility
Molecular polarity plays a crucial role in determining a substance’s solubility in different solvents. Polar substances tend to dissolve in polar solvents, while nonpolar substances dissolve in nonpolar solvents. This principle finds applications in various fields, including pharmaceuticals, cosmetics, and environmental science.
Importance in Biological Systems
Molecular polarity is vital in biological systems, as it affects the interactions between molecules and their effectiveness in performing biological functions. For example, the polarity of water molecules enables them to form hydrogen bonds, which play a crucial role in DNA structure, protein folding, and many other biological processes.
Polarity and Boiling Point
Molecular polarity influences the boiling point of substances. Generally, polar compounds have higher boiling points compared to nonpolar compounds. This is because the intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, are stronger in polar molecules, requiring more energy to break the bonds and transition to the gaseous phase.
Role in Chemical Reactions
The polarity of molecules influences the rate and outcome of chemical reactions. Polar molecules tend to interact more readily with other polar molecules or ions, facilitating the formation of new compounds. Nonpolar solvents, on the other hand, are often used to dissolve nonpolar reactants to promote specific reactions.
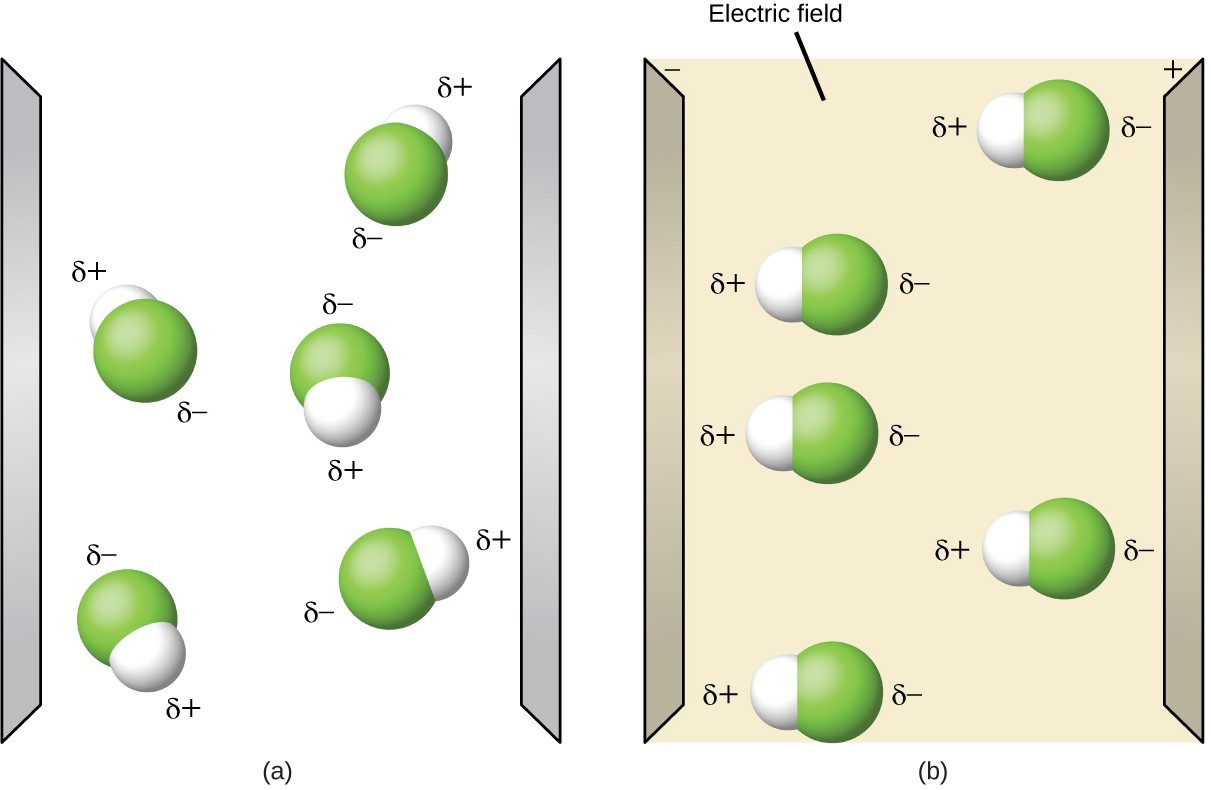
Impact on Electrical Conductivity
Polar substances, when dissolved in a solvent, can conduct electricity due to the movement of charged particles. This is because the polar molecules separate into positive and negative ions, allowing the flow of electric current. In contrast, nonpolar substances do not dissociate into ions and are typically poor conductors of electricity.
Polar vs. Nonpolar Molecules
Polar and nonpolar molecules have distinctive properties. Polar molecules have a dipole moment, exhibit greater intermolecular forces, have higher boiling points, and can form hydrogen bonds. Nonpolar molecules lack a dipole moment, have weaker intermolecular forces, lower boiling points, and do not form hydrogen bonds.
Polarity and Atmospheric Phenomena
Molecular polarity plays a role in atmospheric phenomena such as the formation of raindrops and the behavior of greenhouse gases. The polarity of water molecules allows them to attract and bind to other water molecules, leading to the formation of raindrops. Greenhouse gases, such as carbon dioxide, with polar bonds contribute to the greenhouse effect.
Importance in Material Science
Molecular polarity is crucial in material science, as it affects the properties and performance of materials. The selection and design of materials used in various industries, such as electronics, polymers, and adhesives, heavily rely on understanding and manipulating molecular polarity to achieve desired properties.
In conclusion, molecular polarity is a fundamental concept in chemistry that has far-reaching implications in various scientific disciplines. Understanding the effects of molecular polarity allows chemists to predict the behavior of substances, design new materials, and unravel the complexities of biological systems. The 10 extraordinary facts about molecular polarity mentioned above provide a glimpse into the intriguing world of this essential concept.
Conclusion
In conclusion, understanding molecular polarity is vital for comprehending the behavior and interactions of chemical compounds. The concept of molecular polarity goes beyond simple chemistry and has profound implications in various fields of science, including biology, pharmacology, and material science.
By understanding the extraordinary facts about molecular polarity, we can gain insights into the unique properties of different substances and their applications. From influencing the solubility of a substance to determining its physical and chemical characteristics, molecular polarity plays a crucial role in shaping the world around us.
Exploring the diverse aspects of molecular polarity not only expands our knowledge of the subject but also deepens our appreciation for the intricate world of chemistry. As researchers continue to make new discoveries, we can look forward to unraveling even more fascinating facts about molecular polarity in the future.
FAQs
Q: What is molecular polarity?
A: Molecular polarity refers to the distribution of electrical charge within a molecule. It is determined by the presence of polar bonds and the overall molecular geometry.
Q: How do you determine if a molecule is polar or nonpolar?
A: To determine if a molecule is polar or nonpolar, you need to consider the symmetry of the molecule and the electronegativity difference between the atoms involved in the bonds. If there is a significant electronegativity difference and an asymmetrical molecular shape, the molecule is likely to be polar.
Q: Can a molecule have polar bonds but be nonpolar overall?
A: Yes, a molecule can have polar bonds but be nonpolar overall if the molecular shape is symmetrical. In this case, the polarities of the bonds cancel out, resulting in a nonpolar molecule.
Q: How does molecular polarity affect the physical properties of a substance?
A: Molecular polarity affects the solubility, boiling point, and melting point of a substance. Polar substances tend to dissolve in other polar solvents, while nonpolar substances are more soluble in nonpolar solvents. Additionally, polar molecules generally have higher boiling and melting points compared to nonpolar molecules.
Q: What are some real-life examples of molecular polarity?
A: Some real-life examples of molecular polarity include water (H2O), which is a polar molecule and exhibits various unique properties due to its polarity. Another example is ethanol (C2H5OH), which is also polar and widely used as a solvent in many industries.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
