When it comes to understanding the intricacies of chemistry, one concept that plays a significant role is polarity. Polarity refers to the distribution of charges within a molecule, determining its overall nature and behavior. It affects everything from the solubility of substances to the way molecules interact with each other. With such importance in the realm of chemistry, it’s essential to delve deeper into this fascinating subject.
In this article, we will explore 17 astounding facts about polarity. From understanding the basic principles of polarity and its role in determining molecular structure to exploring the effects of polarity on physical and chemical properties, we will uncover intriguing insights into this essential concept. So whether you’re a chemistry enthusiast or simply eager to expand your knowledge, get ready to be amazed by the captivating world of polarity!
Key Takeaways:
- Understanding polarity is crucial in chemistry, influencing properties like solubility and reactivity. It’s like a magnet guiding chemical interactions!
- Polarity impacts everything from molecule behavior to material design. It’s the secret sauce behind diverse applications in science and engineering!
Polarity is a measure of an atom’s or molecule’s separation of electric charge.
In other words, it describes the distribution of electron density in a chemical species.
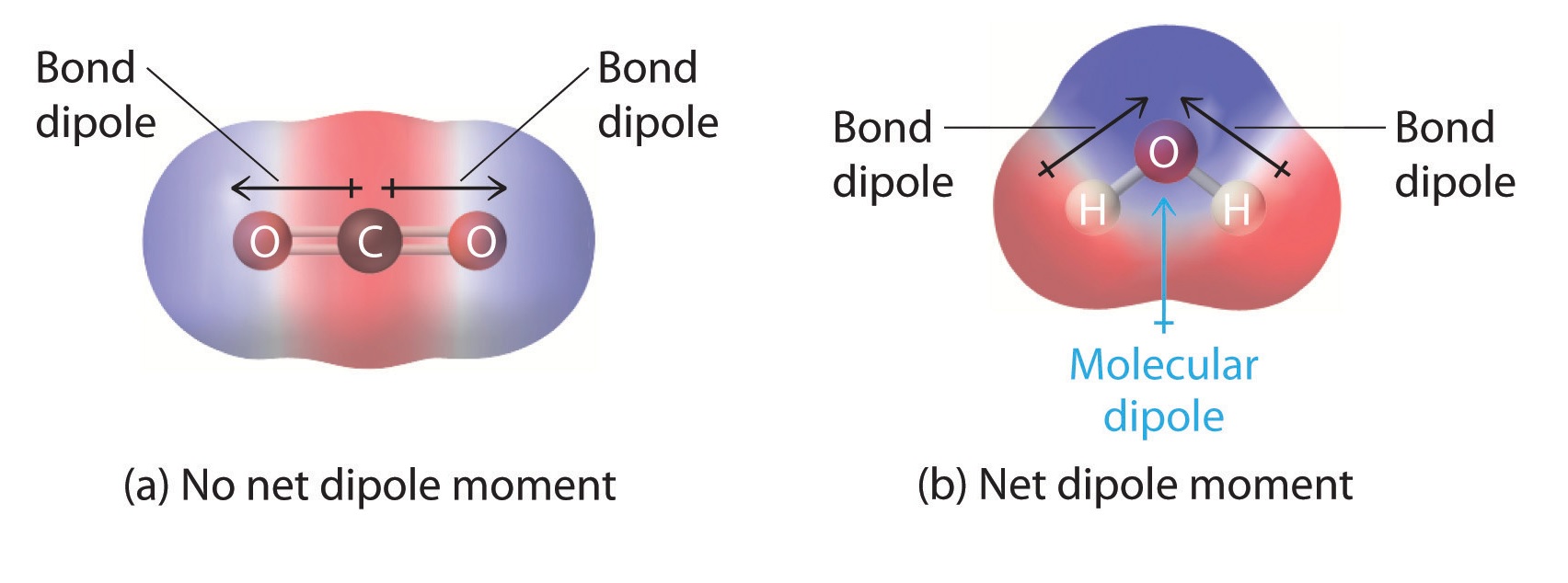
Polar molecules have an uneven distribution of electrons, creating positive and negative poles.
Water (H2O) is a classic example of a polar molecule due to the electronegativity difference between oxygen and hydrogen atoms.
Nonpolar molecules have an even distribution of electrons, resulting in no distinct positive or negative poles.
Examples include carbon dioxide (CO2) and methane (CH4).
Polarity affects a molecule’s physical properties such as boiling point, melting point, and solubility.
Polar substances tend to have higher boiling points and melting points compared to nonpolar substances.
The polarity of a bond is determined by the difference in electronegativity between the atoms involved.
If the electronegativity difference is greater than 0.4, the bond is considered polar.
Electronegativity is a measure of the ability of an atom to attract electrons towards itself.
The higher the electronegativity, the stronger the atom’s pull on electrons.
A polar covalent bond is formed when two atoms with differing electronegativities share electrons unequally.
One atom will have a partial positive charge (?+) and the other a partial negative charge (?-).
Dipole moments are used to measure the polarity of a molecule.
A dipole moment is the product of the magnitude of the charge and the distance of separation between the charges.
The dipole moment is represented by an arrow pointing from the positive to the negative end of a polar molecule.
This arrow indicates the direction of the electron flow.
Polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
This principle forms the basis of the like-dissolves-like rule in solubility.
Polarity is crucial in determining a molecule’s reactivity in chemical reactions.
Electrophiles are attracted to regions of high electron density (negative charge), while nucleophiles are attracted to regions of low electron density (positive charge).
Polarity influences the intermolecular forces between molecules.
Stronger intermolecular forces are present in polar substances, leading to higher boiling and melting points.
Polarity plays a vital role in biological systems.
Cell membranes, for example, are composed of lipids with polar heads and nonpolar tails, allowing for selective transport of molecules.
Polarity in a compound can affect its pharmacological properties.
The polarity of a drug can influence its absorption, distribution, metabolism, and excretion in the body.
The concept of polarity extends beyond individual bonds and molecules.
It applies to overall molecular structures, such as determining the polarity of organic compounds or the polarity of crystals.
Polarity can be influenced by external factors such as temperature and pressure.
Changes in these conditions can alter the distribution of electron density within a molecule.
Understanding polarity is essential in fields such as material science and engineering.
Polar or nonpolar materials are used in various applications, from designing electronic devices to creating waterproof fabrics.
These 17 astounding facts about polarity highlight its significance in the world of chemistry and its diverse applications. Whether you’re studying organic chemistry, analyzing biological systems, or exploring new materials, a solid understanding of polarity is essential for success.
So, embrace the polarity and delve deeper into the captivating world of chemical interactions!
Conclusion
In conclusion, polarity is a fascinating concept in the field of chemistry. It plays a crucial role in understanding the behavior of molecules, chemical reactions, and even the properties of substances. From its impact on solubility and intermolecular forces to its role in determining the shape and structure of molecules, polarity is a fundamental concept that permeates through various branches of chemistry.
By understanding polarity, scientists and researchers can make breakthroughs in areas such as drug development, material science, and environmental studies. The ability to manipulate and harness the polarity of molecules opens up possibilities for creating new compounds, designing more effective drugs, and developing innovative materials with unique properties. The study of polarity continues to inspire exploration and discovery in the quest to understand and harness the fundamental building blocks of matter.
FAQs
1. What is polarity in chemistry?
Polarity refers to the uneven distribution of electron density within a molecule, resulting in the presence of partially positive and partially negative ends. This concept is key in understanding the behavior of molecules and their interactions with other substances.
2. How is polarity determined?
Polarity is determined by factors such as electronegativity and molecular geometry. Electronegativity measures an atom’s ability to attract electrons towards itself, while molecular geometry considers the arrangement of atoms and lone pairs, leading to the overall molecular polarity.
3. Why is polarity important in chemistry?
Polarity plays a crucial role in various chemical processes. It influences the solubility of substances, the strength of intermolecular forces, and the behavior of molecules in reactions. Polarity also determines the physical and chemical properties of substances, such as boiling point, melting point, and polarity-based separations.
4. How does polarity affect bonding?
In polar covalent bonding, electrons are unequally shared between atoms, resulting in partial positive and partial negative charges. In ionic bonding, one or more electrons are completely transferred from one atom to another, leading to the formation of ions with opposite charges. Both types of bonding are influenced by the polarity of the participating atoms.
5. Can polarity be altered?
While the polarity of a molecule is determined by its chemical structure, it can be altered by various methods such as changing the temperature or applying an external electric field. Additionally, certain chemical reactions can introduce functional groups that affect the overall polarity of a molecule.
Polarity's astounding facts merely scratch the surface of this captivating subject. Delving deeper into polarization reveals even more fascinating insights, while molecular polarity holds extraordinary secrets waiting to be uncovered. For those craving a change of pace, polar climates offer astonishing revelations that will leave you eager to explore further.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
