Galvanic cells, also known as voltaic cells, are fascinating electrochemical devices that convert chemical energy into electrical energy. They have played a crucial role in countless scientific experiments, technological advancements, and everyday applications. From powering electronic devices to fuel cells, galvanic cells have proven to be a vital component in various industries.
In this article, we will uncover 9 enigmatic facts about galvanic cells that will give you a deeper understanding of their inner workings and the role they play in chemistry and energy production. We will explore the principles behind galvanic cells, the important components that make them function, and some intriguing applications that have revolutionized industries across the globe.
So, fasten your seatbelts, and get ready to delve into the fascinating world of galvanic cells!
Key Takeaways:
- The galvanic cell is the foundation of batteries, powering everything from smartphones to electric vehicles, and it all starts with its ability to convert chemical energy into electrical energy.
- Galvanic cells are not just for powering devices; they can also be used as sensors to detect pH levels, gases, and even in medical diagnostics, showcasing their versatility and importance in our daily lives.
The galvanic cell is the foundation of batteries.
The galvanic cell serves as the basic building block for various types of batteries, including alkaline, lead-acid, and lithium-ion batteries. Its ability to convert chemical energy into electrical energy makes it an essential component of portable power sources.
The invention of the galvanic cell revolutionized our understanding of electricity.
Italian physicist Alessandro Volta developed the first true galvanic cell in 1800, providing the first practical source of continuous electrical current. This breakthrough paved the way for significant advancements in fields such as telecommunications, transportation, and electronics.
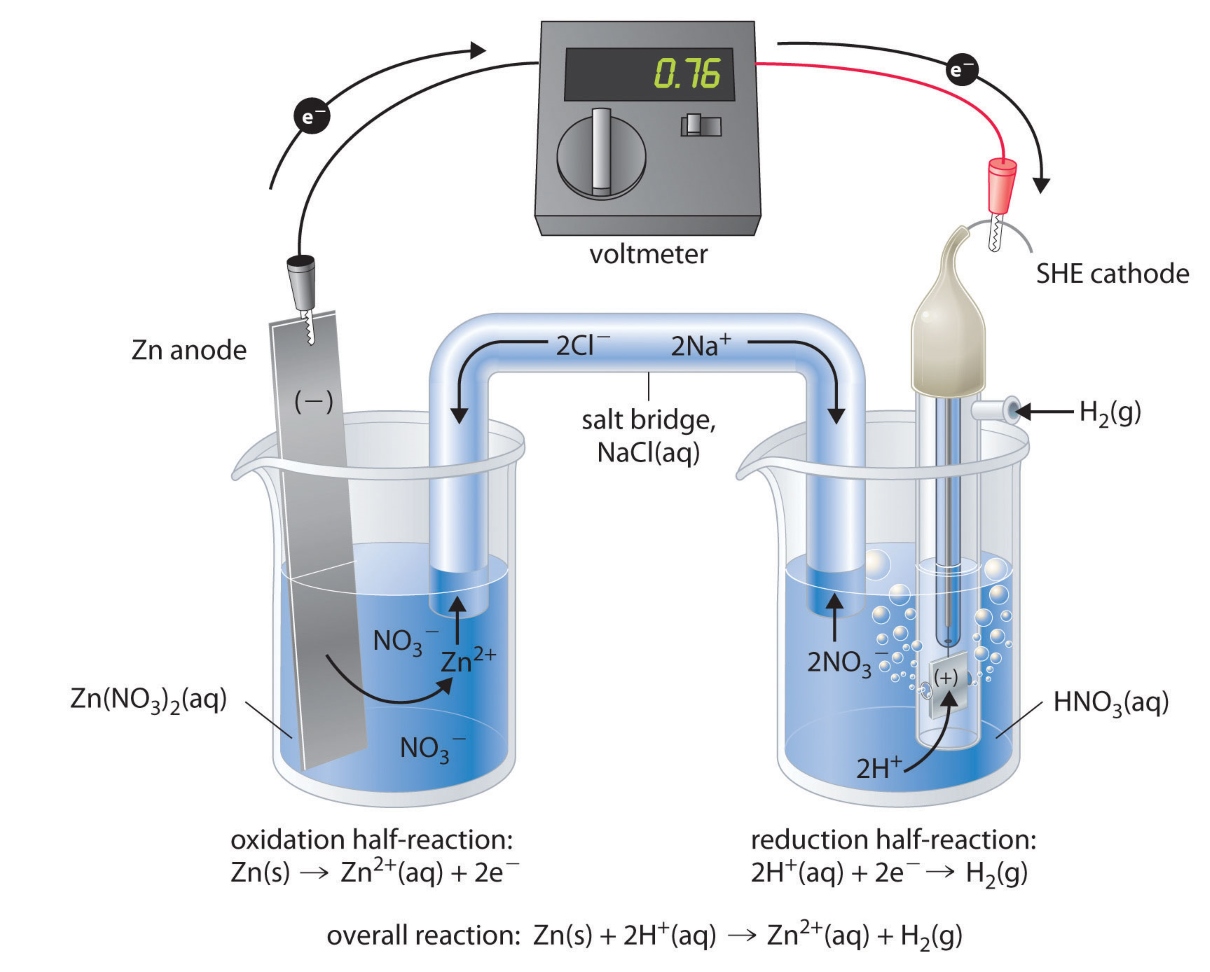
The galvanic cell operates based on redox reactions.
The galvanic cell harnesses the power of oxidation-reduction (redox) reactions to generate electricity. In this process, electrons flow from the anode (the negative electrode) to the cathode (the positive electrode), producing a current that can be utilized for various purposes.
The electrolyte in a galvanic cell is crucial for its operation.
The electrolyte, typically a solution containing ions, plays a vital role in facilitating the flow of ions between the electrodes, maintaining charge balance, and allowing the cell to generate electrical current. Common electrolytes include salt solutions and acidic or alkaline solutions.
Gilbert Lewis developed the concept of electron transfer in galvanic cells.
American chemist Gilbert Lewis introduced the concept of electron transfer between the electrodes of a galvanic cell in the early 20th century. His groundbreaking work laid the foundation for our understanding of how galvanic cells produce electricity.
Galvanic cells can be used as sensors.
By exploiting the electrochemical reactions occurring within the cell, galvanic cells have found applications as sensors for detecting various analytes. These include pH sensors, gas sensors, and biosensors, making them invaluable tools in fields such as environmental monitoring and medical diagnostics.
The galvanic cell can be reversible in certain cases.
Under specific conditions, a galvanic cell can function in reverse, converting electrical energy into chemical energy. This phenomenon is known as an electrolytic cell and is widely used in processes such as electroplating, metal refining, and electrolysis.
The voltage of a galvanic cell depends on the specific redox reactions involved.
Each galvanic cell has a characteristic voltage, also known as its electromotive force (EMF). The EMF is determined by the nature of the redox reactions taking place within the cell and can be used to predict the cell’s ability to generate electrical current.
The galvanic cell has numerous practical applications.
From powering electronic devices to storing renewable energy, galvanic cells play a crucial role in our daily lives. They are used in everything from smartphones and laptops to electric vehicles and renewable energy systems, highlighting their importance in modern society.
Conclusion
In conclusion, galvanic cells are truly fascinating and enigmatic devices that play a crucial role in our everyday lives. From powering portable electronics to being essential components in chemical processes, they are the backbone of many applications in modern technology and industry.Through this article, we have explored nine intriguing facts about galvanic cells. We have learned about their fundamental principles, the different types of cells, and their applications in various fields. We have also discovered the significance of electrode reactions, the role of electrolytes, and the importance of balancing redox reactions.Understanding the inner workings of galvanic cells not only enhances our knowledge of chemistry but also deepens our appreciation for the wonders of electricity. Whether it’s harnessing renewable energy sources or developing innovative battery technologies, galvanic cells continue to revolutionize our world.So, the next time you charge your smartphone or marvel at the awe-inspiring capabilities of electric vehicles, remember the galvanic cell, silently working its magic behind the scenes.
FAQs
1. What is a galvanic cell?
A galvanic cell is an electrochemical device that converts chemical energy into electrical energy through a spontaneous redox reaction.
2. How does a galvanic cell work?
A galvanic cell consists of two half-cells connected by a salt bridge or a porous barrier. In one half-cell, oxidation occurs, generating electrons. In the other half-cell, reduction takes place, accepting the electrons. The flow of electrons through an external circuit generates electrical energy.
3. What is the difference between a galvanic cell and an electrolytic cell?
A galvanic cell produces electrical energy from a spontaneous redox reaction, while an electrolytic cell uses electrical energy to drive a non-spontaneous redox reaction.
4. What are the applications of galvanic cells?
Galvanic cells are used in various applications, such as batteries for portable electronics, electric vehicles, and backup power systems. They are also employed in industrial processes like electroplating and corrosion protection.
5. How long can a galvanic cell last?
The lifespan of a galvanic cell depends on factors such as the type of cell, the nature of the electrodes, and the specific application. Some galvanic cells may last for several years, while others may require periodic replacement.
6. Can galvanic cells be recharged?
Not all galvanic cells can be recharged. Primary cells are non-rechargeable, meaning they cannot be reversed and reused. However, secondary cells, such as lithium-ion batteries, can be recharged by reversing the redox reactions.
7. Are galvanic cells environmentally friendly?
Galvanic cells can be more environmentally friendly compared to other energy sources, especially if they are used in rechargeable battery systems. However, the disposal and recycling of certain battery chemistries can still pose environmental challenges.
8. How can I calculate the voltage of a galvanic cell?
The voltage of a galvanic cell can be calculated using the Nernst equation, which takes into account the standard electrode potentials and the concentration of reactants and products.
9. Are galvanic cells safe?
Galvanic cells can be safe if handled properly. However, certain battery chemistries may pose safety risks if mishandled or subjected to extreme conditions. Following manufacturer guidelines and practicing proper battery handling and disposal is essential.
Galvanic cells' enigmatic nature sparks curiosity, but there's more to explore in the realm of science. Dive into the fascinating world of corrosion, where metals face their ultimate challenge. Electrolyte solutions hold the key to understanding galvanic cells' inner workings, while electrochemistry unveils a treasure trove of surprising facts. Embark on a journey through these captivating topics and expand your knowledge beyond the boundaries of galvanic cells.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
