The Arrhenius Equation is a fundamental concept in chemistry that explains the temperature dependence of reaction rates. Developed by Swedish scientist Svante Arrhenius in 1889, this equation plays a crucial role in various fields of chemistry, including chemical kinetics, thermodynamics, and materials science. It provides a mathematical relationship between the rate constant of a reaction and the temperature at which it occurs.
Understanding the Arrhenius Equation is essential for predicting and controlling the rate of chemical reactions. It has practical applications in industries such as pharmaceuticals, food, and energy, where reaction rates greatly influence process efficiency and product quality.
In this article, we will delve into the fascinating world of the Arrhenius Equation and explore eight intriguing facts that highlight its significance and practical implications. From its derivation to its impact on the speed of chemical reactions, these facts will shed light on the remarkable insights it provides to chemists and scientists alike.
Key Takeaways:
- The Arrhenius Equation, created by a Swedish chemist, helps us understand how temperature affects the speed of chemical reactions. It’s like a special math formula for predicting reaction rates when things get hot!
- This equation is super important in chemistry and other fields like making medicines and designing materials. It helps scientists figure out the best conditions for reactions and understand how temperature changes things.
The Arrhenius Equation is a fundamental tool in chemical kinetics
The Arrhenius Equation, formulated by Swedish chemist Svante Arrhenius in 1889, is a fundamental equation used to describe the temperature dependence of reaction rates in chemical kinetics.
It relates the rate constant of a reaction to temperature
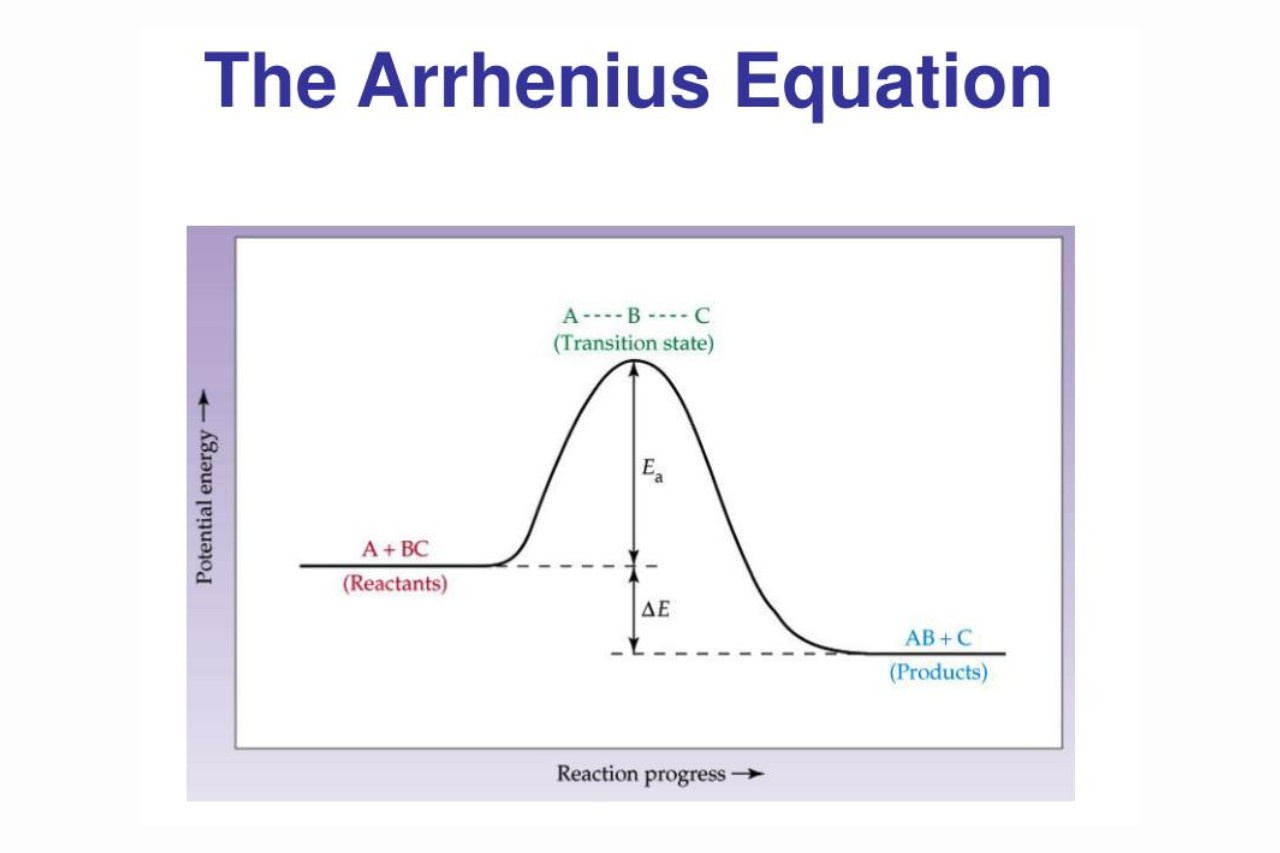
The Arrhenius Equation mathematically relates the rate constant (k) of a reaction to the temperature (T) and the activation energy (Ea) of the reaction. It is expressed as:
k = Ae^(-Ea/RT)
Where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin.
It explains the effect of temperature on reaction rates
The Arrhenius Equation provides a quantitative explanation for the observation that the rate of a chemical reaction generally increases with temperature. According to the equation, as temperature increases, the exponential term in the equation becomes larger, leading to a higher rate constant and faster reaction rate.
The Arrhenius Equation is applicable to various chemical reactions
The Arrhenius Equation can be used to describe the temperature dependence of reaction rates in a wide range of chemical reactions, including both homogeneous and heterogeneous reactions. It is particularly useful in understanding and predicting the behavior of reactions in industrial processes.
The Arrhenius Equation assumes a simple collision model
The Arrhenius Equation is based on the assumption that the reaction rate is determined by the frequency of collisions between the reactant molecules. It assumes a simple collision model where only a fraction of collisions possess the necessary activation energy to result in a reaction.
The Arrhenius Equation has limitations
While the Arrhenius Equation is widely used in chemical kinetics, it does have limitations. It assumes a constant activation energy throughout the reaction and neglects any complex reaction mechanisms or intermediate steps. Additionally, it may not hold true for reactions occurring under extreme conditions.
The Arrhenius Equation can be used to determine activation energies
By measuring the rate constants of a reaction at different temperatures, the Arrhenius Equation can be rearranged to determine the activation energy of the reaction. This information is crucial for understanding the reaction mechanism and designing optimal reaction conditions.
The Arrhenius Equation is essential in many fields of chemistry
The Arrhenius Equation has widespread applications in various fields of chemistry, including chemical engineering, pharmaceuticals, materials science, and environmental science. It plays a crucial role in modeling and optimizing chemical reactions and understanding temperature-dependent processes.
Conclusion
The Arrhenius Equation is a fundamental concept in chemistry that revolutionized the way we understand reaction rates. By incorporating temperature as a crucial factor, the equation provides valuable insights into the kinetics of chemical reactions. Through a deeper understanding of the Arrhenius Equation, scientists and researchers can predict and control reaction rates, enabling the development of new materials, drugs, and industrial processes.
By exploring the 8 fascinating facts about the Arrhenius Equation, we have uncovered the significance and wide-ranging applications of this fundamental equation. From understanding the temperature dependence of reaction rates to its role in climate science and modeling, the Arrhenius Equation continues to shape our understanding of chemical kinetics. So next time you encounter this equation in your studies or research, remember its impact and the exciting possibilities it holds.
FAQs
Q: What is the Arrhenius Equation?
A: The Arrhenius Equation is a mathematical equation that relates the rate constant of a chemical reaction to temperature. It is commonly used to predict how reaction rates change with temperature.
Q: Who discovered the Arrhenius Equation?
A: The Arrhenius Equation is named after Svante Arrhenius, a Swedish scientist who formulated the equation in the late 19th century.
Q: What are the key components of the Arrhenius Equation?
A: The Arrhenius Equation consists of several key components, including the rate constant (k), the pre-exponential factor (A), the activation energy (Ea), the gas constant (R), and the absolute temperature (T).
Q: How does the Arrhenius Equation help predict reaction rates?
A: The Arrhenius Equation allows scientists to quantify the effect of temperature on reaction rates. By measuring the rate constant at different temperatures, the equation can determine the activation energy and predict how reaction rates will change with temperature.
Q: What are some practical applications of the Arrhenius Equation?
A: The Arrhenius Equation has numerous applications in various fields, including chemistry, materials science, pharmacology, and industrial processes. It plays a crucial role in optimizing reaction conditions, understanding thermal degradation, and developing temperature-dependent models.
Q: How does the Arrhenius Equation relate to climate science?
A: The Arrhenius Equation is relevant to climate science as it helps explain the relationship between temperature and the rate of chemical reactions, including those involved in climate change processes, such as the greenhouse gas effect.
Q: Can the Arrhenius Equation be applied to all chemical reactions?
A: While the Arrhenius Equation is a useful tool for many chemical reactions, it may not be applicable to all. Some reactions may deviate from the assumptions made in the Arrhenius Equation, requiring alternative models or approaches.
Q: How has the Arrhenius Equation advanced our understanding of chemical kinetics?
A: The Arrhenius Equation has provided significant insights into the temperature-dependence of reaction rates and has helped scientists develop more accurate models for predicting and controlling chemical reactions. It has enhanced our understanding of reaction mechanisms, enzyme kinetics, and thermal stability of various substances.
Unraveling the mysteries of chemical reactions, the Arrhenius Equation serves as a gateway to understanding chemical kinetics. Temperature's profound influence on reaction rates becomes clear through this powerful tool. Beyond its fundamental role, the equation's applications extend to determining activation energies, making it indispensable across chemistry's diverse landscape.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
