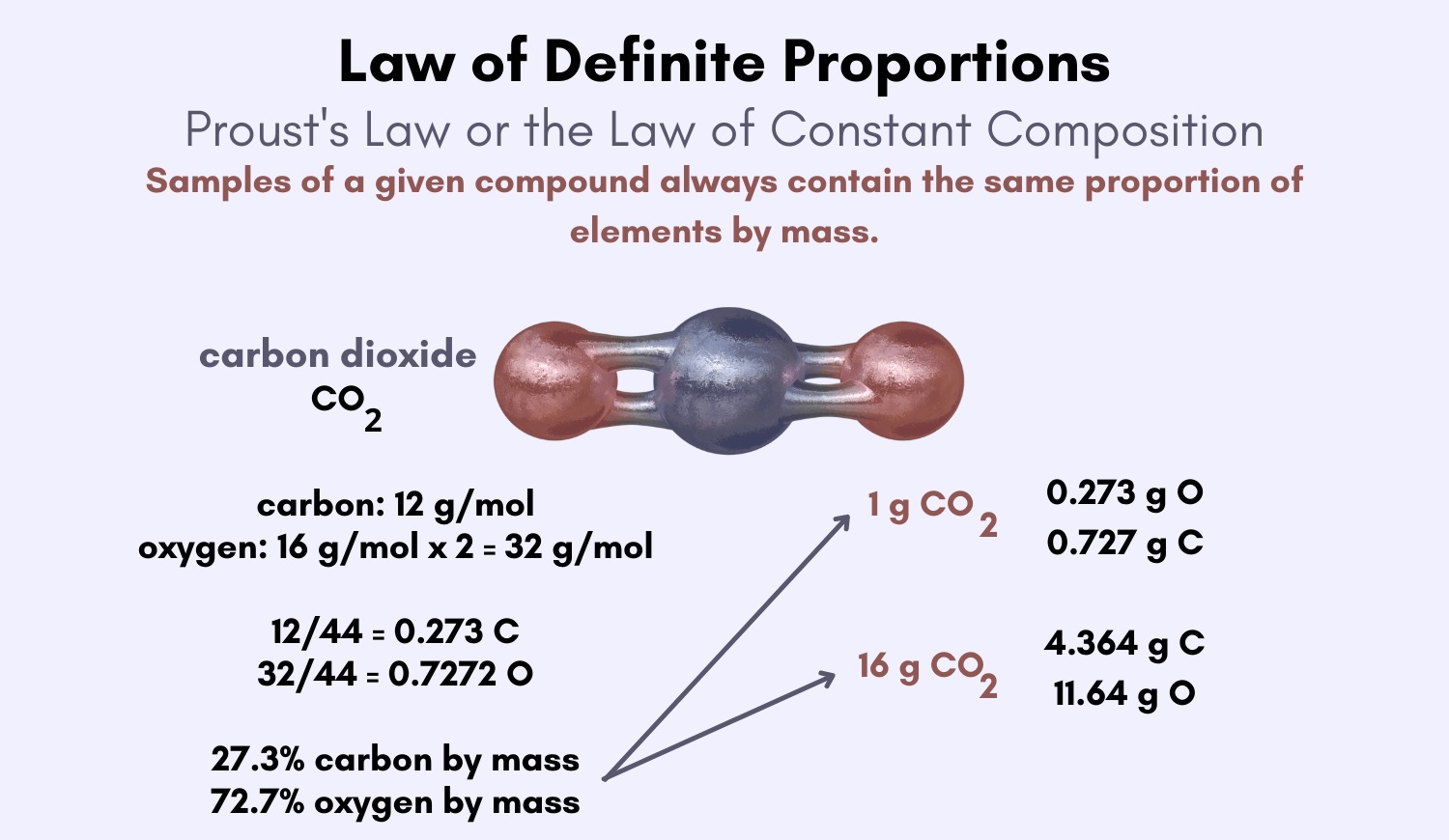
The Law of Definite Proportions, also known as the Law of Constant Composition, is a fundamental principle in chemistry that states that a given chemical compound always contains the same elements in the same proportion by mass. This law, discovered by French chemist Joseph Louis Proust in the late 18th century, revolutionized our understanding of chemical reactions and provided a basis for the development of atomic theory.
The Law of Definite Proportions helps chemists determine the composition of compounds and predict their behavior. By understanding this principle, scientists have been able to unravel the mysteries of chemical reactions and unlock the secrets of the elements that make up our world.
In this article, we will delve deeper into this captivating law and explore eight fascinating facts that will enhance your understanding of the Law of Definite Proportions. So, let’s embark on this chemical journey and uncover the secrets of constant composition!
Key Takeaways:
- The Law of Definite Proportions states that compounds always have the same elements in fixed proportions, which helps chemists predict reactions and identify unknown compounds.
- This law, pioneered by Joseph Proust, is crucial for understanding chemical composition, supporting the periodic table, and aiding in crystallography.
The Foundation of Chemistry
The Law of Definite Proportions, also known as the Law of Constant Composition, is one of the fundamental principles in chemistry. It states that a chemical compound always contains the same elements in fixed proportions by mass, regardless of the source or method of preparation. This law provides a solid foundation for understanding the composition and behavior of compounds in the field of chemistry.
Pioneered by Joseph Proust
The Law of Definite Proportions was first formulated by Joseph Proust, a French chemist, in the late 18th century. Through extensive experiments, Proust discovered that different samples of a compound always had the same ratio of elements and therefore concluded that the ratio of elements in a compound is fixed. His groundbreaking work laid the groundwork for further advancements in chemical analysis and atomic theory.
Implication for Stoichiometry
The Law of Definite Proportions plays a crucial role in stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions. By knowing the fixed proportions of elements in a compound, chemists can predict the amount of each element involved in a reaction and determine the proper ratios needed for a balanced chemical equation.
Key to Identifying Unknown Compounds
The Law of Definite Proportions is a valuable tool for identifying unknown compounds. By analyzing the mass ratio of the elements present in a sample, chemists can compare it to known compound ratios and make accurate determinations about its composition. This information is vital in fields such as forensic science, environmental analysis, and pharmaceutical research.
Supported by Experimental Evidence
The Law of Definite Proportions has been extensively tested and supported by countless experiments. Researchers have analyzed various compounds and consistently found that the mass ratios of their constituent elements remain constant. This empirical evidence reinforces the validity and reliability of this fundamental law in the field of chemistry.
Law of Multiple Proportions
The Law of Definite Proportions is closely related to the Law of Multiple Proportions. The Law of Multiple Proportions states that when two elements form more than one compound, the ratios of the masses of the second element that combine with a fixed mass of the first element can be expressed in small whole numbers. This concept further expands our understanding of the relationships between elements and compounds.
Application in Crystallography
The Law of Definite Proportions finds application in crystallography, the study of crystal structures. By analyzing the arrangement of atoms or molecules in a crystal lattice, scientists can deduce the stoichiometry and proportions of elements within the crystal. This knowledge is crucial for understanding the properties and behavior of various crystals, such as gemstones and minerals.
Fundamental to Periodic Table
The Law of Definite Proportions forms one of the fundamental principles that underpin the organization of the periodic table. The fixed ratios of elements in compounds contribute to the atomic masses and properties assigned to each element. This understanding of elemental composition greatly aids in the classification and study of chemical elements and their interactions.
Conclusion
The Law of Definite Proportions is a fundamental principle in chemistry that states that a chemical compound always contains the same elements in fixed and definite proportions by mass. This law was first formulated by Joseph Proust in the late 18th century and has since played a crucial role in our understanding of chemical reactions and composition.
By studying the Law of Definite Proportions, chemists have been able to make significant advancements in various fields, including pharmaceuticals, materials science, and environmental chemistry. This principle allows scientists to accurately predict the outcome of chemical reactions and determine the composition of compounds, leading to the development of new drugs, stronger and more durable materials, and effective pollution control mechanisms.
Understanding and applying the Law of Definite Proportions is essential for any student or professional in the field of chemistry. By grasping this concept, we can uncover the fascinating world of chemical reactions and explore the intricacies of matter.
FAQs
1. What is the Law of Definite Proportions?
The Law of Definite Proportions states that a chemical compound always contains the same elements in fixed and definite proportions by mass.
2. Who discovered the Law of Definite Proportions?
The Law of Definite Proportions was first formulated by Joseph Proust, a French chemist, in the late 18th century.
3. Why is the Law of Definite Proportions important?
This law is important because it allows chemists to predict the outcome of chemical reactions and determine the composition of compounds accurately. It has played a crucial role in various scientific advancements, including drug development, materials science, and pollution control.
4. How does the Law of Definite Proportions contribute to pharmaceutical advancements?
By understanding the Law of Definite Proportions, scientists can precisely determine the composition of drugs, ensuring their efficacy and safety. This knowledge helps in designing new drugs with specific chemical compositions and properties.
5. Can the Law of Definite Proportions be applied to all chemical compounds?
Yes, the Law of Definite Proportions applies to all chemical compounds, regardless of their complexity. It is a fundamental principle that governs the composition of matter.
Exploring the Law of Definite Proportions is just the beginning of your journey into the fascinating world of chemistry. Dive deeper into the captivating realm of chemistry facts, unravel the enigmatic nature of stoichiometry, and prepare to be amazed by the unbelievable truths behind chemical bonding. Each topic offers a unique perspective on the building blocks of our universe, promising to expand your knowledge and ignite your curiosity. So, what are you waiting for? Choose your path and embark on an adventure through the captivating landscape of chemistry!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
