Arsenic acid might sound like something out of a mad scientist's lab, but it’s actually a fascinating compound with a rich history and diverse uses. Did you know that this chemical has been used in everything from agriculture to medicine? Arsenic acid is a powerful substance that can be both beneficial and dangerous, depending on how it’s handled. In this blog post, we’ll explore 40 intriguing facts about arsenic acid that will surprise you. From its role in ancient civilizations to its modern applications, there’s a lot to learn about this unique compound. Ready to dive in? Let’s get started!
Key Takeaways:
- Arsenic acid is a versatile compound used in various industries, but it poses serious health and environmental risks. Safety measures and proper handling are crucial to prevent harm.
- Despite its historical significance and industrial importance, arsenic acid's toxicity requires careful handling and disposal to protect human health and the environment.
What is Arsenic Acid?
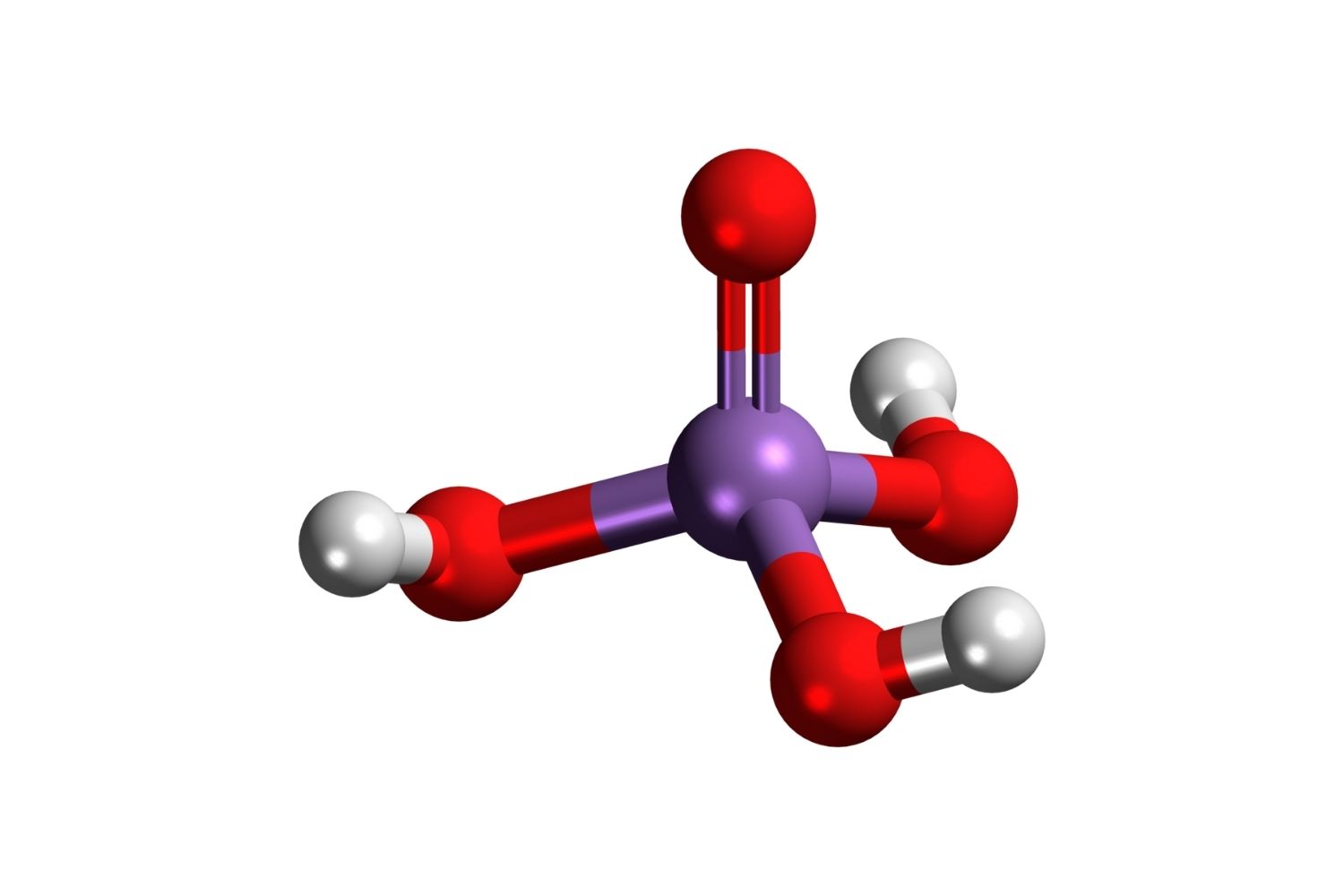
Arsenic acid is a chemical compound with the formula H₃AsO₄. It’s an important substance in various industrial processes and has significant historical relevance. Here are some intriguing facts about arsenic acid.
-
Arsenic acid is a weak acid, similar to phosphoric acid in structure and properties.
-
It is typically found in a white, crystalline form that is highly soluble in water.
-
This compound is often used in the production of insecticides and herbicides.
-
Arsenic acid can also be used in the glass industry to remove bubbles from molten glass.
-
Historically, it was used in the preservation of wood, although this practice has declined due to its toxicity.
Chemical Properties of Arsenic Acid
Understanding the chemical properties of arsenic acid helps in grasping its applications and risks. Here are some key chemical characteristics.
-
Arsenic acid has three acidic protons, making it a triprotic acid.
-
It can form salts known as arsenates when it reacts with bases.
-
The compound decomposes upon heating, releasing toxic arsenic fumes.
-
Arsenic acid is a strong oxidizing agent, capable of converting other substances to their oxidized forms.
-
It reacts with metals to form arsenate salts and hydrogen gas.
Uses of Arsenic Acid
Arsenic acid has diverse applications across various industries. Here are some notable uses.
-
It is used in the manufacturing of semiconductors.
-
The compound serves as a desiccant in the production of certain dyes.
-
Arsenic acid is employed in the synthesis of organic arsenic compounds.
-
It plays a role in the production of lead hydrogen arsenate, an insecticide.
-
The compound is used in the treatment of leather to prevent mold and mildew.
Health Effects of Arsenic Acid
Exposure to arsenic acid can have serious health implications. Here are some facts about its effects on health.
-
Arsenic acid is highly toxic and can cause severe poisoning if ingested.
-
Chronic exposure can lead to skin lesions, cancer, and cardiovascular diseases.
-
Inhalation of arsenic acid fumes can cause respiratory issues and lung cancer.
-
It can also affect the nervous system, leading to symptoms like headaches and confusion.
-
Arsenic acid is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC).
Environmental Impact of Arsenic Acid
The environmental impact of arsenic acid is significant due to its toxicity and persistence. Here are some facts about its environmental effects.
-
Arsenic acid can contaminate soil and water, posing risks to plants and animals.
-
It can accumulate in the food chain, leading to higher concentrations in predators.
-
The compound is resistant to degradation, making it a long-term pollutant.
-
Arsenic acid can disrupt aquatic ecosystems by affecting the growth and reproduction of aquatic organisms.
-
It can also affect the microbial communities in soil, altering nutrient cycling processes.
Safety Measures for Handling Arsenic Acid
Given its toxicity, proper safety measures are essential when handling arsenic acid. Here are some important safety tips.
-
Always wear protective clothing, gloves, and eye protection when handling arsenic acid.
-
Use fume hoods or other ventilation systems to avoid inhaling fumes.
-
Store arsenic acid in a cool, dry place away from incompatible substances.
-
Dispose of arsenic acid waste according to local regulations to prevent environmental contamination.
-
In case of exposure, seek immediate medical attention and follow first aid procedures.
Historical Facts about Arsenic Acid
Arsenic acid has a rich history, with various uses and discoveries over the centuries. Here are some historical facts.
-
Arsenic compounds have been used since ancient times, often for medicinal purposes.
-
In the 19th century, arsenic acid was used in the production of green pigments for wallpapers and fabrics.
-
The compound played a role in the development of early pesticides and herbicides.
-
Arsenic acid was once used in the treatment of syphilis before the advent of antibiotics.
-
The infamous poisonings in the Victorian era often involved arsenic compounds, including arsenic acid.
Interesting Facts about Arsenic Acid
Here are some additional interesting facts about arsenic acid that you might not know.
-
Arsenic acid can be produced by the oxidation of arsenic trioxide.
-
It has a melting point of 35.5°C (95.9°F) and a boiling point of 160°C (320°F).
-
The compound can form hydrates, with the most common being the hemihydrate (H₃AsO₄·0.5H₂O).
-
Arsenic acid is used in some analytical chemistry techniques to detect the presence of certain metals.
-
Despite its toxicity, arsenic acid continues to be an important chemical in various industrial processes.
Final Thoughts on Arsenic Acid
Arsenic acid, a compound with a notorious reputation, has a fascinating array of uses and dangers. It's found in pesticides, wood preservatives, and even some industrial processes. However, its toxic nature makes it a double-edged sword. Exposure can lead to severe health issues, including cancer and organ damage.
Understanding arsenic acid's properties and risks is crucial for safe handling. Always follow safety guidelines and use protective gear when dealing with this substance. Awareness and caution can prevent harmful exposure and ensure safety in environments where arsenic acid is present.
In summary, while arsenic acid has practical applications, its potential hazards can't be ignored. Stay informed, stay safe, and respect the power of this potent chemical.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
