VSEPR Theory, or Valence Shell Electron Pair Repulsion Theory, helps us understand the shapes of molecules. Ever wondered why water has a bent shape or why methane forms a tetrahedron? VSEPR Theory explains it all! This theory states that electron pairs around a central atom will arrange themselves to be as far apart as possible, minimizing repulsion. This simple idea can predict complex molecular geometries. From linear to trigonal bipyramidal, the shapes are fascinating. Whether you're a student, a teacher, or just curious, these 34 facts will make VSEPR Theory crystal clear. Ready to dive into the world of molecular shapes? Let's get started!
Understanding VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) theory helps predict the shape of molecules. This theory is essential for understanding molecular geometry and chemical bonding. Here are some fascinating facts about VSEPR theory.
- VSEPR theory was developed by Ronald Gillespie and Ronald Nyholm in the 1950s.
- The theory is based on the idea that electron pairs around a central atom will repel each other.
- Electron pairs include both bonding pairs and lone pairs.
- Bonding pairs are shared between atoms, forming a bond.
- Lone pairs are not shared and belong to a single atom.
- The shape of a molecule is determined by the number of bonding and lone pairs around the central atom.
- Linear geometry occurs when there are two bonding pairs and no lone pairs.
- Trigonal planar geometry happens with three bonding pairs and no lone pairs.
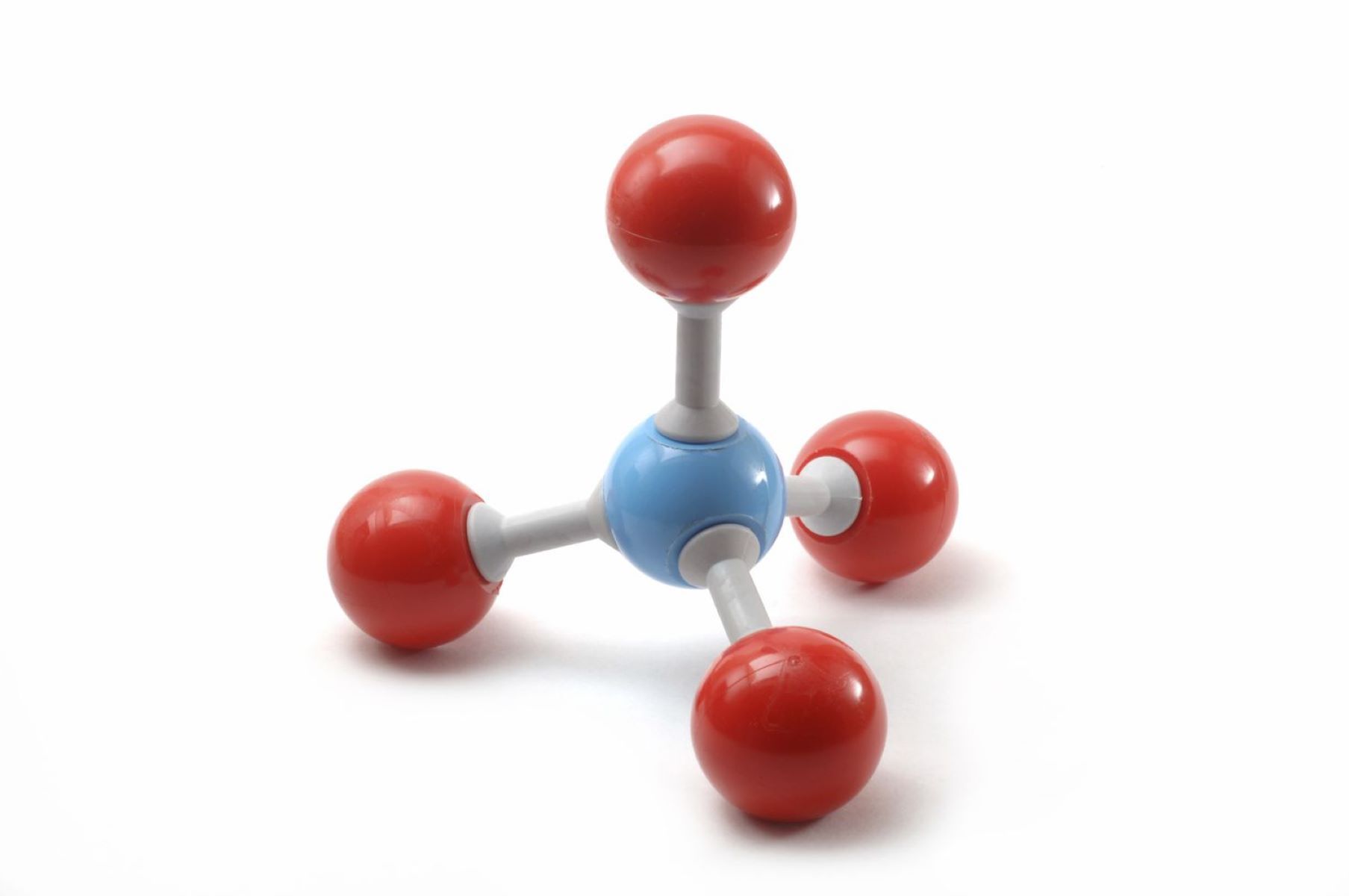
- Tetrahedral geometry forms with four bonding pairs and no lone pairs.
- Trigonal bipyramidal geometry appears with five bonding pairs and no lone pairs.
- Octahedral geometry is seen with six bonding pairs and no lone pairs.
Importance of Lone Pairs
Lone pairs play a crucial role in determining the shape of molecules. They occupy more space than bonding pairs, affecting the angles between bonds.
- Lone pairs cause bond angles to be smaller than expected.
- Water (H2O) has a bent shape due to two lone pairs on the oxygen atom.
- Ammonia (NH3) has a trigonal pyramidal shape because of one lone pair on the nitrogen atom.
- Sulfur hexafluoride (SF6) has an octahedral shape with no lone pairs on the sulfur atom.
- Methane (CH4) has a tetrahedral shape with no lone pairs on the carbon atom.
- Phosphorus pentachloride (PCl5) has a trigonal bipyramidal shape with no lone pairs on the phosphorus atom.
Predicting Molecular Shapes
VSEPR theory helps predict the shapes of various molecules, which is crucial for understanding their properties and reactivity.
- Carbon dioxide (CO2) has a linear shape with two double bonds and no lone pairs on the carbon atom.
- Boron trifluoride (BF3) has a trigonal planar shape with three bonding pairs and no lone pairs on the boron atom.
- Xenon tetrafluoride (XeF4) has a square planar shape with four bonding pairs and two lone pairs on the xenon atom.
- Chlorine trifluoride (ClF3) has a T-shaped geometry with three bonding pairs and two lone pairs on the chlorine atom.
- Iodine pentafluoride (IF5) has a square pyramidal shape with five bonding pairs and one lone pair on the iodine atom.
Applications of VSEPR Theory
Understanding molecular shapes is vital in various fields, including chemistry, biology, and materials science.
- Drug design relies on VSEPR theory to predict how molecules will interact with biological targets.
- Catalysis involves understanding the shapes of catalyst molecules to improve their efficiency.
- Material science uses VSEPR theory to design new materials with specific properties.
- Environmental science applies VSEPR theory to understand pollutant behavior and interactions.
- Nanotechnology benefits from VSEPR theory in designing nanoscale structures.
Limitations of VSEPR Theory
While VSEPR theory is useful, it has some limitations and cannot predict everything about molecular shapes.
- VSEPR theory does not account for the effects of d-orbitals in transition metals.
- The theory assumes that all electron pairs repel equally, which is not always true.
- It cannot accurately predict the shapes of molecules with multiple central atoms.
- VSEPR theory does not consider the influence of molecular orbitals on shape.
- The theory is less accurate for large, complex molecules.
Fun Facts about VSEPR Theory
Here are some interesting tidbits that make VSEPR theory even more fascinating.
- VSEPR theory is sometimes called the Gillespie-Nyholm theory after its developers.
- The theory is widely taught in high school and college chemistry courses worldwide.
The Final Takeaway
Understanding VSEPR Theory can really change how you see molecules. It’s all about how electron pairs repel each other, shaping the molecule. This theory helps predict shapes like linear, trigonal planar, and tetrahedral. Knowing these shapes can explain why water has a bent shape or why carbon dioxide is linear.
Remember, lone pairs take up more space than bonding pairs. This affects bond angles, making them smaller than expected. VSEPR Theory isn’t just for chemists; it’s useful in fields like biology and materials science too.
By grasping these concepts, you’ll have a better handle on molecular geometry. This knowledge can help in understanding reactions, properties, and functions of different substances. So, keep these facts in mind next time you’re studying molecules. It’s a small step that can lead to big insights in science.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


