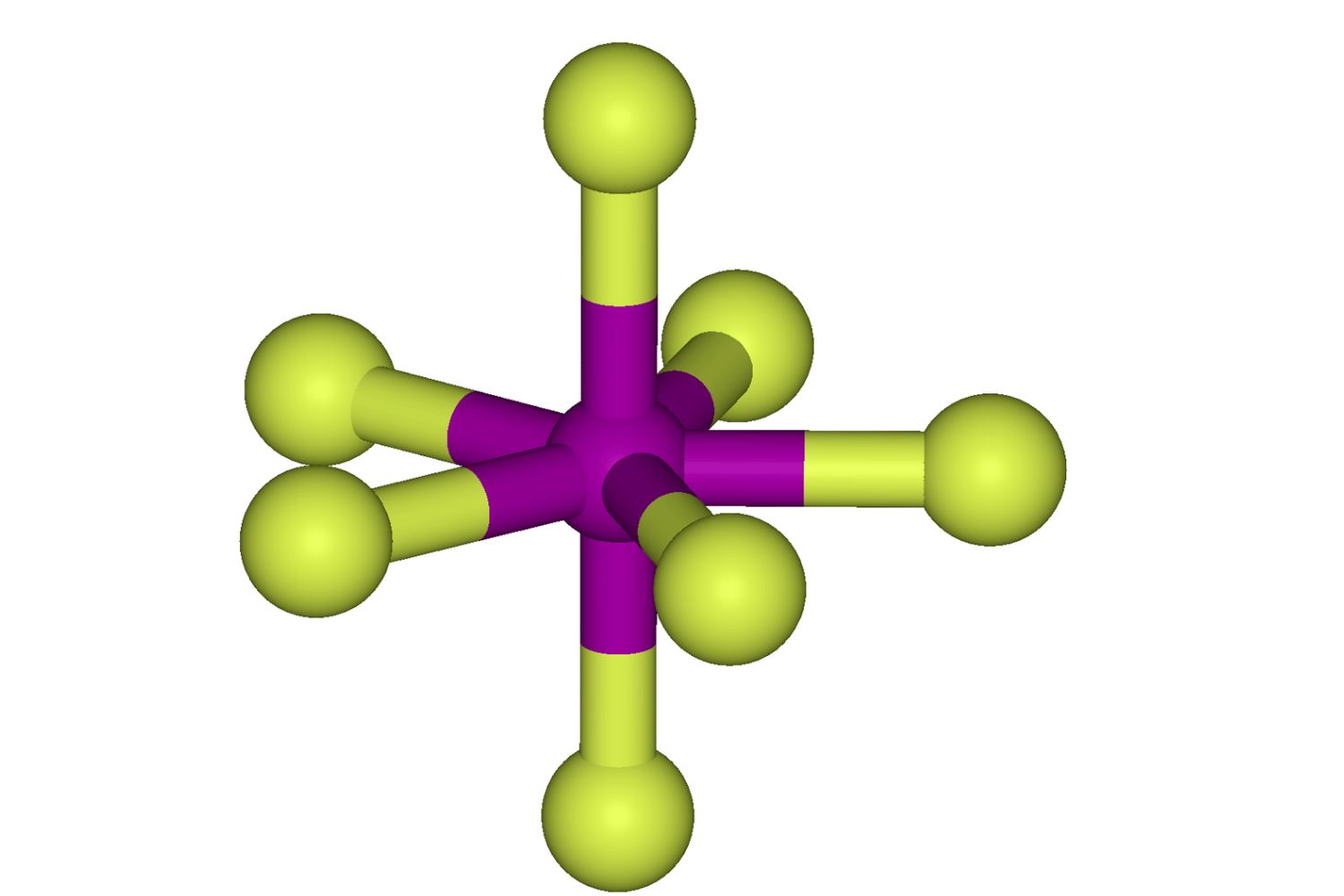
Iodine heptafluoride might sound like a mouthful, but it's a fascinating chemical compound with some surprising properties. Did you know that iodine heptafluoride, also known as IF7, is one of the few compounds where iodine exhibits its highest oxidation state? This colorless gas is highly reactive and used in various industrial applications. But what makes it so special? For starters, its unique molecular structure features iodine at the center surrounded by seven fluorine atoms, forming a pentagonal bipyramidal shape. This configuration gives it some unusual characteristics, such as being a powerful fluorinating agent. Curious about more? Let's dive into 30 intriguing facts about iodine heptafluoride that will expand your understanding of this remarkable compound.
Key Takeaways:
- Iodine Heptafluoride (IF7) is a unique and highly reactive gas with applications in the semiconductor industry, nuclear fuel processing, and organic synthesis. It requires careful handling due to its corrosive nature and potential environmental impact.
- IF7, a colorless gas with a pungent odor, is the only iodine fluoride that is a gas at room temperature. It has a pentagonal bipyramidal molecular structure and can form complexes with other molecules, making it a versatile compound with both practical and hazardous properties.
What is Iodine Heptafluoride?
Iodine Heptafluoride, also known as IF7, is a chemical compound with some fascinating properties. This compound is a colorless gas at room temperature and has a unique molecular structure. Let's dive into some intriguing facts about Iodine Heptafluoride.
-
IF7 is the only iodine fluoride that is a gas at room temperature. Most other iodine fluorides are either liquids or solids under the same conditions.
-
The molecular geometry of IF7 is pentagonal bipyramidal. This means it has seven fluorine atoms arranged around a central iodine atom in a unique shape.
-
IF7 is highly reactive. It can react with many substances, including water, to form hydrofluoric acid and iodine pentoxide.
-
It was first synthesized in 1963. The compound was created by Neil Bartlett, a chemist known for his work with noble gases.
-
IF7 is used in the semiconductor industry. Its reactivity makes it useful for etching silicon wafers.
Chemical Properties of Iodine Heptafluoride
Understanding the chemical properties of IF7 can help us appreciate its applications and behavior in different environments.
-
IF7 is a strong oxidizing agent. It can easily accept electrons from other substances, making it useful in various chemical reactions.
-
It has a high boiling point of 4.8°C. Despite being a gas at room temperature, it doesn't take much to condense it into a liquid.
-
IF7 is non-polar. The symmetrical arrangement of fluorine atoms around the iodine atom cancels out any dipole moments.
-
It can form complexes with other molecules. For example, it can react with Lewis bases to form adducts.
-
IF7 is corrosive. It can cause severe burns upon contact with skin or other tissues.
Uses of Iodine Heptafluoride
Despite its reactivity and potential hazards, IF7 has several practical applications.
-
Used in nuclear fuel processing. IF7 can help in the separation of uranium from spent nuclear fuel.
-
Employed in organic synthesis. It can introduce fluorine atoms into organic molecules, which can alter their properties.
-
Serves as a fluorinating agent. IF7 can add fluorine to other compounds, making it useful in various chemical industries.
-
Used in the production of fluoropolymers. These materials have applications in non-stick coatings and other high-performance materials.
-
Helps in the preparation of other iodine fluorides. IF7 can be a starting material for synthesizing other iodine-fluorine compounds.
Safety and Handling of Iodine Heptafluoride
Given its reactivity and corrosiveness, proper safety measures are crucial when working with IF7.
-
IF7 must be stored in airtight containers. This prevents it from reacting with moisture in the air.
-
Personal protective equipment (PPE) is essential. Gloves, goggles, and protective clothing can help prevent burns and other injuries.
-
Proper ventilation is necessary. Working with IF7 in a fume hood can help avoid inhalation of harmful gases.
-
Emergency procedures should be in place. Quick access to eyewash stations and safety showers can mitigate accidents.
-
Training is crucial. Anyone handling IF7 should be well-versed in its properties and potential hazards.
Environmental Impact of Iodine Heptafluoride
Understanding the environmental impact of IF7 can help in developing better handling and disposal methods.
-
IF7 can contribute to air pollution. Its reactivity means it can form harmful byproducts when released into the atmosphere.
-
It can contaminate water sources. If IF7 comes into contact with water, it can form hydrofluoric acid, which is highly toxic.
-
Proper disposal is essential. IF7 should be neutralized and disposed of according to local regulations to minimize environmental harm.
-
Monitoring is necessary. Regular checks can help ensure that IF7 is not leaking or being released into the environment.
-
Research is ongoing. Scientists are continually studying IF7 to better understand its environmental impact and find safer handling methods.
Interesting Facts about Iodine Heptafluoride
Here are some lesser-known but equally fascinating facts about IF7.
-
IF7 is colorless but has a pungent odor. This makes it easy to detect leaks through smell.
-
It is one of the few compounds where iodine exhibits a +7 oxidation state. This is the highest oxidation state iodine can achieve.
-
IF7 can be synthesized by direct fluorination of iodine. This involves reacting iodine with fluorine gas under controlled conditions.
-
It has a high electron affinity. This means it can easily attract electrons from other substances.
-
IF7 is not found naturally. It is a man-made compound, synthesized for specific industrial and research purposes.
Final Thoughts on Iodine Heptafluoride
Iodine heptafluoride is a fascinating compound with unique properties. Its role in chemical synthesis and industrial applications makes it an important substance in various fields. Understanding its reactivity and handling precautions is crucial for safe usage. This compound's ability to act as a strong fluorinating agent opens doors to numerous chemical reactions, making it valuable for researchers and industry professionals alike.
While iodine heptafluoride may not be a household name, its significance in scientific and industrial contexts cannot be overstated. Whether you're a chemistry enthusiast or a professional in the field, knowing these facts about iodine heptafluoride can enhance your appreciation for this remarkable compound. Stay curious and keep exploring the wonders of chemistry!
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
