Bromine trifluoride might sound like a fancy chemical name, but it's actually a fascinating compound with some pretty cool properties. What is bromine trifluoride used for? Bromine trifluoride is primarily used as a powerful fluorinating agent and in nuclear fuel processing. This means it helps add fluorine to other substances, which is super useful in making certain chemicals and materials. It's also known for its ability to dissolve many metals and even some rocks, making it handy in industrial settings. But don't let its usefulness fool you—it's highly reactive and needs careful handling. Imagine a liquid that can ignite on contact with water! That's bromine trifluoride for you. Its unique characteristics make it a valuable tool in chemistry, but also a reminder of the power and potential risks of chemical reactions. Whether you're a budding scientist or just curious, bromine trifluoride is a compound worth knowing about.
Key Takeaways:
- Bromine Trifluoride, or BrF3, is a powerful and highly reactive chemical compound used in industries for uranium enrichment, chemical synthesis, and even as a potential rocket propellant.
- Handling Bromine Trifluoride requires strict safety measures due to its toxicity, reactivity with water, and corrosive nature. Its unique properties make it both fascinating and hazardous in the world of chemistry.
What is Bromine Trifluoride?
Bromine Trifluoride, often abbreviated as BrF3, is a chemical compound that might not be a household name, but it plays a significant role in various scientific and industrial applications. Let's dive into some intriguing facts about this fascinating compound.
-
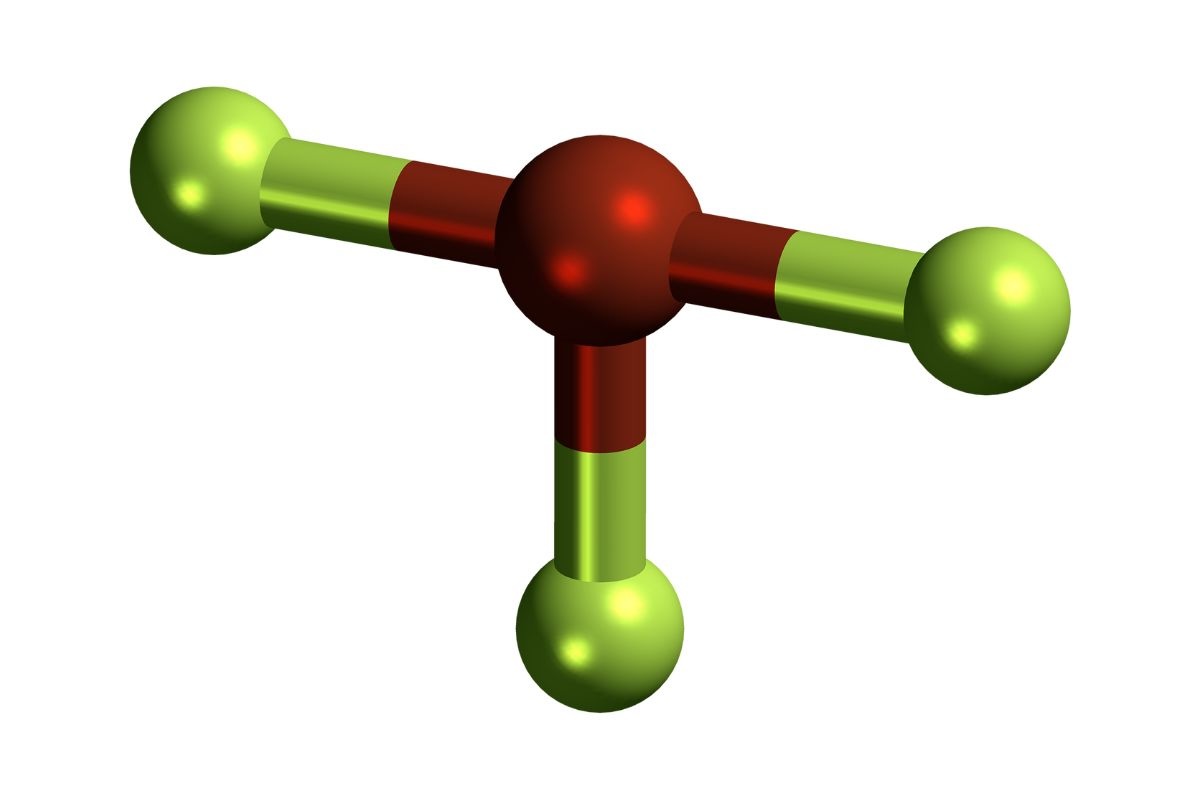
Chemical Formula: BrF3 stands for Bromine Trifluoride, a compound consisting of one bromine atom and three fluorine atoms. This combination gives it unique properties.
-
Color and State: At room temperature, Bromine Trifluoride is a pale yellow liquid. Its color can be quite striking, especially when compared to other chemicals.
-
Highly Reactive: This compound is known for its extreme reactivity. It can react with almost any substance, including water, metals, and even glass.
-
Powerful Fluorinating Agent: In the world of chemistry, BrF3 is a powerful fluorinating agent. It can add fluorine atoms to other molecules, which is useful in various chemical reactions.
-
Corrosive Nature: Bromine Trifluoride is highly corrosive. It can eat through many materials, making it a challenging substance to handle safely.
-
Industrial Uses: Despite its reactivity, BrF3 is used in industries for processes like uranium enrichment and the production of certain chemicals.
-
Handling Precautions: Due to its corrosive and reactive nature, handling BrF3 requires special precautions, including protective gear and proper ventilation.
How is Bromine Trifluoride Produced?
The production of Bromine Trifluoride involves specific chemical reactions and conditions. Understanding its production helps appreciate its complexity and usefulness.
-
Direct Fluorination: Bromine Trifluoride is produced by the direct reaction of bromine with fluorine gas. This process requires careful control of conditions to ensure safety and efficiency.
-
Temperature Control: The reaction to produce BrF3 is exothermic, meaning it releases heat. Controlling the temperature is crucial to prevent unwanted side reactions.
-
Purity Matters: The purity of the bromine and fluorine used in the reaction affects the quality of the Bromine Trifluoride produced. Impurities can lead to less effective or even dangerous products.
What are the Applications of Bromine Trifluoride?
Despite its hazardous nature, Bromine Trifluoride finds applications in various fields due to its unique properties.
-
Nuclear Industry: In the nuclear industry, BrF3 is used for uranium enrichment, a critical process in producing nuclear fuel.
-
Chemical Synthesis: It serves as a reagent in chemical synthesis, helping to create complex molecules by adding fluorine atoms.
-
Cleaning Agent: Surprisingly, BrF3 is used to clean certain metal surfaces by removing oxides and other impurities.
-
Rocket Propellant: In some cases, Bromine Trifluoride has been explored as a potential rocket propellant due to its high energy release upon decomposition.
-
Etching Agent: In the electronics industry, BrF3 is used as an etching agent to create intricate patterns on semiconductor materials.
What are the Safety Concerns with Bromine Trifluoride?
Handling Bromine Trifluoride requires strict safety measures due to its hazardous nature. Understanding these concerns is vital for anyone working with this compound.
-
Toxicity: BrF3 is highly toxic if inhaled or ingested. It can cause severe respiratory and skin irritation.
-
Reactivity with Water: When Bromine Trifluoride comes into contact with water, it reacts violently, releasing toxic gases like hydrogen fluoride.
-
Storage Challenges: Storing BrF3 requires special containers that can withstand its corrosive nature. Glass and many metals are unsuitable for storage.
-
Emergency Protocols: Facilities using BrF3 must have emergency protocols in place to handle accidental releases or spills safely.
-
Environmental Impact: If released into the environment, Bromine Trifluoride can cause significant harm to ecosystems due to its reactivity and toxicity.
What are the Chemical Properties of Bromine Trifluoride?
The chemical properties of Bromine Trifluoride make it a unique compound with specific behaviors and reactions.
-
Molecular Geometry: BrF3 has a T-shaped molecular geometry, which influences its reactivity and interactions with other molecules.
-
Bond Angles: The bond angles in Bromine Trifluoride are approximately 86.2 degrees, contributing to its unique shape and properties.
-
Polar Molecule: Due to its shape and the difference in electronegativity between bromine and fluorine, BrF3 is a polar molecule.
-
Boiling Point: Bromine Trifluoride has a boiling point of 126.8 degrees Celsius, which is relatively high for a compound of its size.
-
Density: The density of BrF3 is about 2.803 g/cm³, making it denser than water.
What are the Historical Aspects of Bromine Trifluoride?
The history of Bromine Trifluoride provides insight into its discovery and development over time.
-
Discovery: BrF3 was first synthesized in the early 20th century by chemists exploring the reactions of halogens.
-
Research Evolution: Over the years, research on Bromine Trifluoride has expanded, leading to a better understanding of its properties and applications.
-
Military Interest: During certain periods, there was military interest in BrF3 due to its potential use in chemical warfare, although this was never fully realized.
-
Scientific Contributions: Studies on Bromine Trifluoride have contributed to the broader understanding of halogen chemistry and reactivity.
-
Continued Exploration: Today, scientists continue to explore new applications and safer handling methods for Bromine Trifluoride, ensuring its place in modern chemistry.
Bromine Trifluoride: A Fascinating Chemical
Bromine trifluoride is a chemical compound that packs a punch. Known for its reactivity and corrosive nature, this compound is a force to be reckoned with in the world of chemistry. Its ability to dissolve glass and other materials makes it a valuable tool in industrial applications. However, handling it requires extreme caution due to its hazardous properties. Despite its dangers, bromine trifluoride plays a crucial role in nuclear fuel processing and chemical synthesis. Its unique characteristics make it a subject of interest for scientists and researchers. Understanding its properties and applications can lead to advancements in various fields. Whether you're a chemistry enthusiast or just curious about the world around you, bromine trifluoride offers a glimpse into the complexity and power of chemical reactions. Stay curious and keep exploring the wonders of science!
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
