Europium(III) iodate might sound like a mouthful, but it's a fascinating compound with some cool properties. Europium, a rare earth element, combines with iodate to form this unique substance. Found in various scientific applications, it plays a role in everything from phosphorescent materials to nuclear reactors. Ever wondered why your TV screen glows so brightly? Europium could be the reason! This compound also has intriguing chemical properties that make it valuable in research. Whether you're a science enthusiast or just curious about the elements, learning about Europium(III) iodate can open up a world of wonder. Ready to dive into some amazing facts? Let's get started!
Key Takeaways:
- Europium(III) Iodate is a rare compound with unique properties, used in TV screens, LED lights, and nuclear science. It glows under UV light and should be handled with care.
- Discovered in 1901, Europium(III) Iodate is a fascinating chemical with high melting point and stability. It doesn't dissolve in water and has potential for new applications.
What is Europium(III) Iodate?
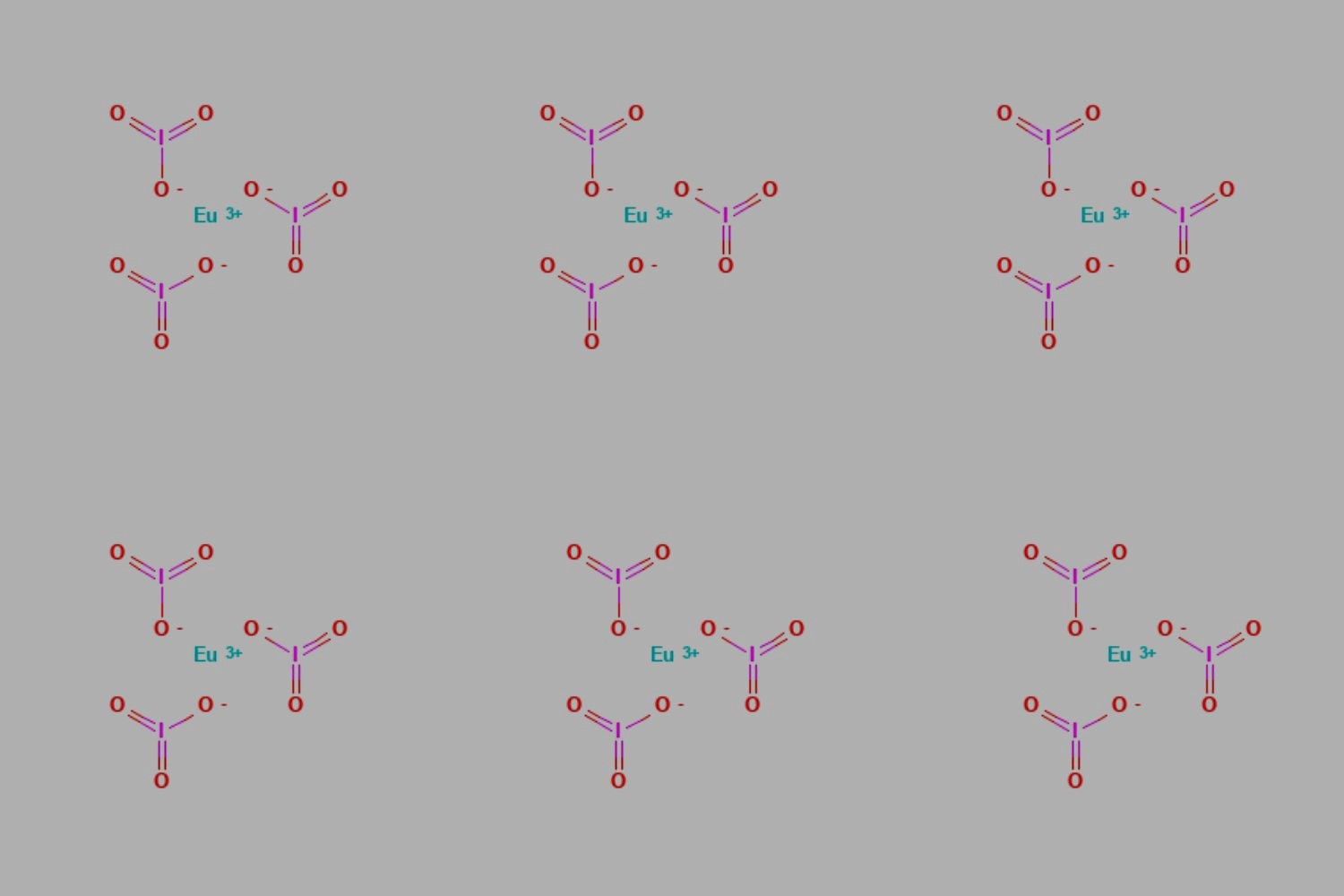
Europium(III) iodate is a chemical compound that combines europium, a rare earth element, with iodate ions. This compound has unique properties and applications, making it an interesting subject for study.
- Europium(III) iodate has the chemical formula Eu(IO3)3.
- It is composed of europium ions (Eu3+) and iodate ions (IO3-).
- Europium belongs to the lanthanide series on the periodic table.
- Iodate ions are derived from iodic acid (HIO3).
Physical Properties of Europium(III) Iodate
Understanding the physical properties of europium(III) iodate can help in identifying its potential uses and behaviors in different environments.
- Europium(III) iodate appears as a white crystalline solid.
- It is insoluble in water, meaning it does not dissolve easily.
- The compound has a high melting point, indicating strong ionic bonds.
- It is stable under normal conditions but can decompose at high temperatures.
Chemical Properties of Europium(III) Iodate
The chemical properties of europium(III) iodate reveal how it reacts with other substances and its stability.
- Europium(III) iodate is an oxidizing agent, meaning it can accept electrons from other substances.
- It reacts with strong acids to form europium salts and iodic acid.
- The compound can be reduced to europium(II) iodate under certain conditions.
- It does not react with water, maintaining its structure in aqueous environments.
Applications of Europium(III) Iodate
Europium(III) iodate has various applications due to its unique properties, particularly in scientific research and technology.
- It is used in the production of phosphors for color television screens and LED lights.
- The compound is valuable in the field of nuclear science for neutron capture studies.
- It serves as a catalyst in certain chemical reactions, speeding up the process without being consumed.
- Europium(III) iodate is also used in the development of specialized glass and ceramics.
Safety and Handling of Europium(III) Iodate
Handling europium(III) iodate requires understanding its safety protocols to prevent any harmful effects.
- The compound should be handled with care, using gloves and protective eyewear.
- Inhalation of its dust can cause respiratory irritation.
- It should be stored in a cool, dry place away from incompatible substances.
- Proper disposal methods must be followed to avoid environmental contamination.
Interesting Facts about Europium(III) Iodate
Here are some intriguing tidbits about europium(III) iodate that highlight its uniqueness and importance.
- Europium was discovered in 1901 by French chemist Eugène-Anatole Demarçay.
- The element europium is named after the continent of Europe.
- Europium(III) iodate can exhibit luminescence, glowing under UV light.
- It is one of the few compounds that contain both a rare earth element and an iodate ion.
- Research is ongoing to explore new applications and properties of europium(III) iodate.
Final Thoughts on Europium(III) Iodate
Europium(III) iodate, a fascinating compound, offers a wealth of intriguing properties and applications. From its role in phosphorescent materials to its use in scientific research, this compound stands out in the world of chemistry. Its unique luminescent properties make it invaluable in various fields, including lighting and display technologies. Additionally, its chemical stability and reactivity provide a rich area for further exploration and innovation.
Understanding europium(III) iodate not only broadens our knowledge of rare earth elements but also opens doors to new technological advancements. Whether you're a chemistry enthusiast or a professional researcher, the study of this compound can lead to exciting discoveries and practical applications. Keep exploring, stay curious, and who knows? You might just uncover the next big breakthrough in the world of europium compounds.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
