Cyanogen thiocyanate might sound like a mouthful, but it's a fascinating compound with some unique properties. What exactly is cyanogen thiocyanate? It's a chemical compound with the formula (SCN)₂. This compound is known for its pungent smell and is used in various chemical reactions. Cyanogen thiocyanate is often employed in organic synthesis, especially in the formation of thiocyanates and isocyanates. It’s also notable for its role in the production of pharmaceuticals and pesticides. Despite its usefulness, it’s crucial to handle it with care due to its toxic nature. Ready to learn more about this intriguing compound? Let's dive into 20 facts that will broaden your understanding of cyanogen thiocyanate.
Key Takeaways:
- Cyanogen Thiocyanate is a versatile compound used in pesticides, disinfectants, and chemical synthesis. It's important to handle it with caution due to its toxic and irritating nature.
- Understanding its chemical reactions and environmental impact is crucial for safe use. Despite its historical use, proper handling and disposal are essential to prevent harm to humans and the environment.
What is Cyanogen Thiocyanate?
Cyanogen thiocyanate is a chemical compound with a unique structure and interesting properties. It's used in various fields, from chemistry to industry. Here are some fascinating facts about this compound.
-
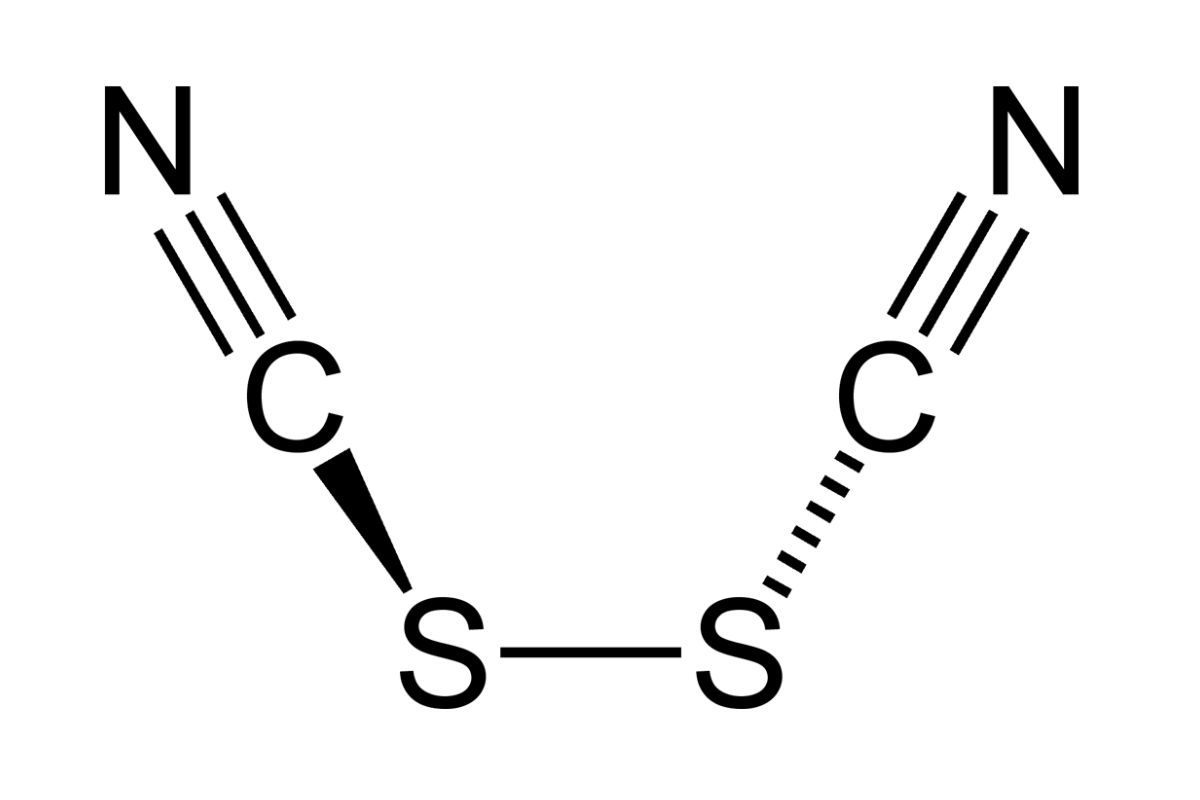
Chemical Formula: Cyanogen thiocyanate has the chemical formula C₂N₂S. This formula indicates it contains two carbon atoms, two nitrogen atoms, and one sulfur atom.
-
Molecular Structure: The molecule consists of a cyanogen group (C≡N) bonded to a thiocyanate group (SCN). This structure gives it distinctive chemical properties.
-
Appearance: In its pure form, cyanogen thiocyanate is a colorless liquid. However, it can appear yellowish due to impurities.
-
Odor: It has a pungent, irritating odor. This smell is a warning sign of its presence, as it can be harmful if inhaled.
Uses of Cyanogen Thiocyanate
This compound has several applications, making it valuable in different industries. Let's explore some of its uses.
-
Pesticide: Cyanogen thiocyanate is used as a pesticide. It helps control pests in agricultural settings, protecting crops from damage.
-
Chemical Synthesis: It's a reagent in organic synthesis. Chemists use it to create various compounds, including pharmaceuticals and dyes.
-
Disinfectant: Due to its antimicrobial properties, cyanogen thiocyanate is used as a disinfectant. It helps kill bacteria and other pathogens on surfaces.
-
Laboratory Research: Researchers use it in experiments to study chemical reactions and properties. Its unique structure makes it a valuable tool in scientific investigations.
Safety and Handling
Handling cyanogen thiocyanate requires caution due to its hazardous nature. Here are some important safety facts.
-
Toxicity: Cyanogen thiocyanate is toxic if ingested, inhaled, or absorbed through the skin. Proper protective equipment is essential when working with it.
-
Irritant: It can irritate the skin, eyes, and respiratory system. Immediate washing and medical attention are necessary if exposure occurs.
-
Storage: Store it in a cool, dry place away from incompatible substances. Proper storage prevents accidental reactions and spills.
-
Disposal: Dispose of cyanogen thiocyanate according to local regulations. Improper disposal can harm the environment and public health.
Chemical Reactions
Cyanogen thiocyanate participates in various chemical reactions. These reactions are crucial for its applications and understanding its behavior.
-
Hydrolysis: When exposed to water, cyanogen thiocyanate hydrolyzes to form hydrogen cyanide and thiocyanic acid. This reaction is important in its use as a pesticide.
-
Oxidation: It can be oxidized to form cyanogen chloride and sulfur dioxide. These products have their own industrial applications.
-
Polymerization: Under certain conditions, cyanogen thiocyanate can polymerize. This reaction changes its properties and can be useful in creating new materials.
Environmental Impact
Understanding the environmental impact of cyanogen thiocyanate is important for its safe use. Here are some key points.
-
Degradation: In the environment, cyanogen thiocyanate degrades into less harmful substances. This process reduces its long-term impact.
-
Bioaccumulation: It does not significantly bioaccumulate in organisms. This property makes it less likely to cause long-term ecological harm.
-
Aquatic Toxicity: Cyanogen thiocyanate is toxic to aquatic life. Careful management is necessary to prevent contamination of water bodies.
Historical Context
The history of cyanogen thiocyanate provides insight into its development and use. Here are some historical facts.
-
Discovery: Cyanogen thiocyanate was first synthesized in the 19th century. Early chemists explored its properties and potential uses.
-
Industrial Use: Its use in industry grew in the 20th century. Advances in chemistry and technology expanded its applications, making it a valuable compound in various fields.
Final Thoughts on Cyanogen Thiocyanate
Cyanogen thiocyanate, a fascinating compound, has a lot to offer. Its unique properties make it valuable in various fields, from organic synthesis to potential medical applications. Understanding its chemical behavior helps scientists develop new materials and drugs. Despite its toxic nature, proper handling and safety measures ensure it can be used effectively without harm.
Learning about cyanogen thiocyanate broadens our knowledge of chemistry and its practical uses. Whether you're a student, researcher, or just curious, knowing these facts can spark interest in further exploration. Keep safety in mind, and you'll find this compound both intriguing and useful.
Thanks for joining us on this journey through the world of cyanogen thiocyanate. Stay curious and keep exploring the wonders of science!
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
