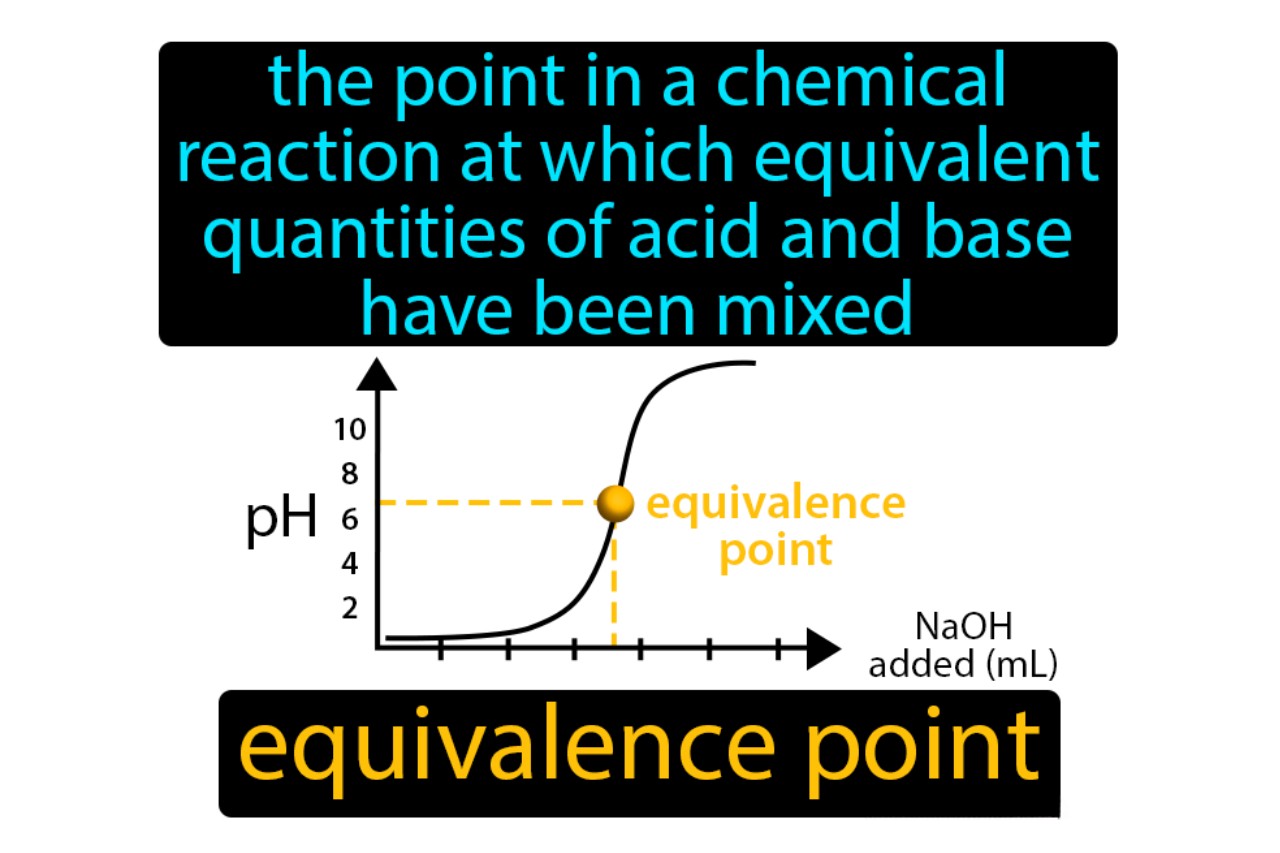
The equivalence point is a fundamental concept in chemistry that plays a crucial role in various chemical reactions and titrations. It is the point at which the stoichiometrically equivalent amounts of reactants have reacted completely to form the desired products.
Understanding the equivalence point is essential for chemists as it allows them to determine the concentration of unknown substances, predict reaction outcomes, and ensure accuracy in experimental procedures. It is a fascinating phenomenon that holds many intriguing facts.
In this article, we will explore 19 captivating facts about the equivalence point, shedding light on its significance, applications, and unique properties. So, let’s dive into the world of chemistry and uncover the mysteries surrounding this fundamental concept.
Key Takeaways:
- The equivalence point is the magical moment in a chemical reaction when all the reactants have turned into products. It’s like reaching the perfect balance in a seesaw!
- By finding the equivalence point, scientists can figure out the concentration of mysterious substances and even solve the mystery of their molar mass. It’s like being a chemistry detective!
The equivalence point is a crucial concept in chemical titrations.
At the equivalence point, the amount of reactant added is stoichiometrically equivalent to the amount of reactant consumed, resulting in the complete conversion of reactants to products.
The equivalence point can be determined using various indicators.
Indicators such as phenolphthalein and bromothymol blue are commonly used to signal the color change that occurs at the equivalence point.
The equivalence point is dependent on the balanced chemical equation of the reaction.
The stoichiometry of the reaction determines the ratio of reactants and products at the equivalence point.
The pH at the equivalence point can vary depending on the reaction.
For acid-base titrations, the pH at the equivalence point is often around 7 for neutralization reactions. However, in other types of titrations, such as oxidation-reduction reactions, the pH can be different.
The equivalence point is crucial for determining the concentration of unknown substances.
By using known concentrations of titrant and measuring the volume required to reach the equivalence point, the concentration of the unknown substance can be calculated using the stoichiometry of the reaction.
The equivalence point can be identified graphically using a titration curve.
A titration curve plots the pH or another property of the solution against the volume of titrant added, allowing for the determination of the equivalence point.
The color change at the equivalence point is not always instantaneous.
Some indicators exhibit a gradual color change over a range of pH values, resulting in a broader range of volumes around the equivalence point.
The equivalence point is also known as the stoichiometric point.
This term emphasizes the balanced ratio of reactants and products at the point of equivalence.
The equivalence point can be determined analytically or visually.
Analytical methods involve measuring changes in electrical conductivity, absorbance, or other properties, while visual methods rely on color changes or other visible indicators.
The equivalence point is essential in determining the endpoint of a titration.
The endpoint is the point at which the indicator changes color, indicating that the equivalence point has been reached.
The pH at the equivalence point depends on the nature of the acid-base reaction.
Strong acid-strong base titrations typically result in a neutral pH, while weak acid-strong base or weak base-strong acid titrations can result in acidic or basic equivalence points.
The equivalence point can be estimated using preliminary calculations.
By understanding the stoichiometry of the reaction and the initial concentrations of the reactants, an estimated equivalence point can be calculated before conducting the titration.
The equivalence point is significant in determining the molar mass of unknown substances.
By knowing the volume and concentration of the titrant at the equivalence point, the molar mass of the unknown substance can be determined using the ideal gas law or other relevant equations.
Different types of titrations have different equivalence points.
Acid-base titrations have an equivalence point characterized by a pH of 7 for neutralization reactions. Redox titrations involve a change in oxidation states and have different equivalence points depending on the specific reaction.
The equivalence point can also be determined using a pH meter.
A pH meter provides a more accurate and precise measurement of the pH at the equivalence point compared to visual indicators.
The location of the equivalence point on a titration curve depends on the strength of the acid and base being titrated.
Strong acid-strong base titrations usually have an equivalence point near the center of the curve, while weak acid-strong base or weak base-strong acid titrations may have an equivalence point shifted towards one end.
The equivalence point is a crucial parameter in determining the equivalence factor of a substance.
The equivalence factor represents the number of moles of a certain substance reacting with one mole of the titrant.
The equivalence point is used to calculate the endpoint of a titration.
The endpoint signifies the completion of the reaction and is usually marked by a color change or other observable indicator.
The equivalence point is an essential concept in analytical chemistry.
It not only helps determine the concentration of unknown substances but also allows for the identification and characterization of various chemical species.
Conclusion
In conclusion, the equivalence point is a key concept in chemistry that is essential for understanding various chemical reactions and analytical techniques. It represents the point at which the stoichiometric amounts of reactants and products are present in a solution. Equivalence points can be determined through different methods, such as titrations and pH measurements, and they provide valuable information about the concentration of substances in a solution.During an equivalence point, various physical and chemical changes occur, leading to the formation of new compounds and the neutralization of acidity or alkalinity. Understanding the equivalence point is crucial for scientists and researchers as it helps in determining reaction kinetics, calculating concentrations, and predicting the outcome of chemical reactions.By delving into the fascinating facts about the equivalence point, we can enhance our understanding of chemical reactions and their applications in everyday life. Whether you’re a student exploring chemistry or a professional in the field, the knowledge of the equivalence point can greatly contribute to your understanding of the subject.So, let’s embrace the richness of the equivalence point concept and continue to explore the thrilling world of chemistry!
FAQs
Q: What exactly is the equivalence point in chemistry?A: The equivalence point is the stage in a chemical reaction where the stoichiometric ratio of the reactants and products are completely balanced, indicating that the reaction is complete.Q: How is the equivalence point determined?A: The equivalence point can be determined through various methods, such as titrations, pH measurements, or visual indicators. These techniques help determine the point at which the required amounts of reactants have reacted completely.Q: What is the significance of the equivalence point?A: The equivalence point provides valuable information about the concentration of substances in a solution and helps in understanding reaction kinetics, calculating concentrations, and predicting the outcome of reactions.Q: Can the equivalence point be exceeded?A: Yes, it is possible to exceed the equivalence point in certain cases. This occurs when excess reactants are present in a reaction, resulting in a higher than expected quantity of products.Q: Are all chemical reactions guaranteed to reach an equivalence point?A: No, not all chemical reactions reach an equivalence point. Some reactions may stop before reaching this point due to limitations such as insufficient reactants, irreversible reactions, or competing chemical reactions.Q: Can the equivalence point be influenced by external factors?A: Yes, external factors such as temperature, pressure, and the presence of catalysts can influence the equivalence point in a chemical reaction. These factors can affect the rate of reaction and the formation of products.
Mastering the equivalence point is just the beginning of your chemistry adventure. Want to take your titration skills to the next level? Check out our mind-blowing facts about titration curves, where you'll learn how to interpret the shape and meaning behind these fascinating graphs. Get ready to impress your lab partners and ace your next chemistry exam!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
