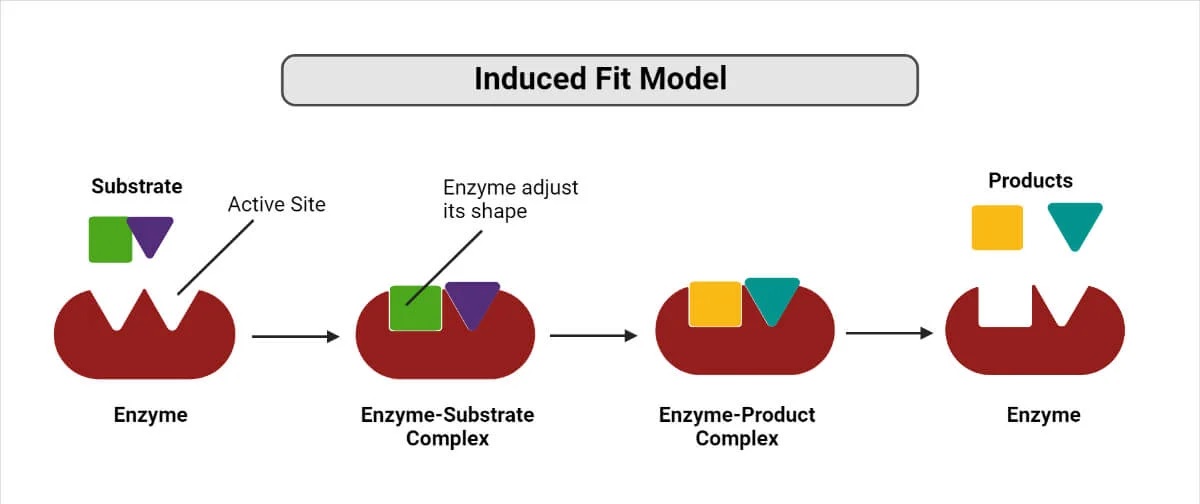
The induced fit model is a fascinating concept in the field of biochemistry that explains how enzymes interact with substrates to carry out essential chemical reactions in living organisms. In this model, the active site of an enzyme undergoes some changes in its conformation upon binding to the substrate, resulting in a better fit between the two molecules. This dynamic interaction between enzymes and substrates plays a crucial role in catalysis and ensures the efficiency and specificity of biochemical reactions.
In this article, we will delve deeper into the intriguing world of the induced fit model and explore 17 astounding facts that highlight its importance and impact in the field of chemistry. From its discovery to its applicability in drug design, these facts will provide a comprehensive understanding of this fundamental concept in biochemistry. So, let’s embark on this scientific journey and uncover the hidden wonders of the induced fit model!
Key Takeaways:
- The induced fit model explains how enzymes and substrates change shape to fit together perfectly, enhancing the efficiency of biochemical reactions.
- Understanding the induced fit model is crucial for developing effective drugs and advancing our knowledge of biochemistry and molecular biology.
What is the Induced Fit Model?
The induced fit model proposes that enzymes and substrates undergo conformational changes upon binding to enhance their interaction and facilitate the formation of the enzyme-substrate complex.
Key Players: Enzyme and Substrate
In the induced fit model, the enzyme refers to the biological molecule that acts as a catalyst, while the substrate is the specific molecule or molecules that the enzyme acts upon.
Dynamic Changes in Shape
Both the enzyme and the substrate undergo dynamic changes in their shapes when they come into contact with each other, allowing for a more precise fit.
Flexible Active Site
The active site of the enzyme, where the substrate binds, is not rigid but rather flexible, allowing it to adjust its shape to accommodate the substrate.
Complementary Shape Changes
The induced fit model suggests that the enzyme and substrate undergo complementary shape changes to achieve optimal binding and catalytic activity.
Lock and Key vs. Induced Fit
The induced fit model contrasts with the lock and key model, which states that the enzyme and substrate have preexisting complementary shapes that fit perfectly together.
Enzyme-Substrate Recognition
The induced fit model explains how enzymes can recognize specific substrates in complex cellular environments and exclude other molecules.
Enhanced Catalytic Efficiency
The induced fit model enhances catalytic efficiency by bringing the enzyme and substrate in closer proximity and reducing the activation energy of the reaction.
Allosteric Effects
The induced fit model is closely associated with allosteric effects, where the binding of a molecule at one site on the enzyme affects the conformation and activity at another site.
Substrate-Induced Conformational Changes
When the substrate binds to the enzyme, it induces conformational changes that optimize the active site for catalysis.
Energy Redistribution
The induced fit model involves energy redistribution within the enzyme-substrate complex, which is vital for the efficient conversion of substrate to product.
Specificity and Selectivity
The induced fit model contributes to the specificity and selectivity of enzymatic reactions by ensuring that only compatible substrates are bound and converted.
Transition State Stabilization
The induced fit model aids in the stabilization of transition state intermediates, which are high-energy states during a reaction that lead to the formation of products.
Multistep Binding Process
The induced fit model involves a multistep binding process, where initial weak interactions between the enzyme and substrate lead to stronger and more specific interactions.
Conformational Flexibility
The induced fit model relies on the ability of both the enzyme and substrate to undergo conformational flexibility, allowing for the necessary interactions to take place.
Relevance in Drug Design
The induced fit model has significant implications in drug design, as understanding the dynamic interactions between enzymes and substrates can aid in the development of specific and effective drugs.
Experimental Evidence
The induced fit model is supported by various experimental techniques such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, which allow for the visualization of enzyme-substrate complexes and conformational changes.
The induced fit model revolutionized our understanding of enzyme-substrate interactions. By incorporating dynamic changes and flexibility into the binding process, it provides a more accurate representation of the complexities involved in enzymatic reactions. With its relevance in various fields, including biochemistry, medicine, and drug design, the induced fit model continues to be a topic of ongoing research and discovery.
Conclusion
In conclusion, the induced fit model is a fascinating concept that explains how enzymes and substrates interact during chemical reactions. This model highlights the dynamic nature of enzyme-substrate interactions, where both the enzyme and substrate undergo conformational changes to form a more optimal fit. By understanding the induced fit model, scientists can better comprehend the intricacies of enzyme catalysis and design more efficient drugs and catalysts.
FAQs
Q: What is the induced fit model?
A: The induced fit model is a theory that explains how enzymes and substrates interact during chemical reactions. According to this model, both the enzyme and substrate undergo conformational changes to achieve a more optimal fit, enhancing the enzyme’s catalytic activity.
Q: How does the induced fit model differ from the lock and key model?
A: Unlike the lock and key model, which suggests that the enzyme and substrate have rigid shapes that perfectly complement each other, the induced fit model emphasizes the flexibility of both the enzyme and substrate. In the induced fit model, the enzyme and substrate undergo structural changes to create a more precise fit.
Q: What is the significance of the induced fit model?
A: The induced fit model provides insights into how enzymes function and catalyze reactions. By understanding the dynamic nature of enzyme-substrate interactions, scientists can better design drugs, enzymes, and catalysts that specifically target certain reactions and improve efficiency.
Q: Can enzymes undergo multiple conformational changes during the induced fit process?
A: Yes, enzymes can undergo multiple conformational changes during the induced fit process. These changes can involve both the active site and other regions of the enzyme, allowing for a more precise and efficient catalytic reaction.
Q: Are all enzymes capable of undergoing induced fit?
A: Yes, most enzymes are capable of undergoing induced fit. However, the extent of conformational changes may vary depending on the specific enzyme and substrate involved in the reaction. Some enzymes may require significant structural rearrangements, while others may undergo minimal changes.
Enzymes' induced fit model is just one piece of the biochemistry puzzle. Dive deeper into how catalysis accelerates chemical reactions, explore protein structure's role in enzyme function, or discover more fascinating biochemistry facts.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
