Electrochemistry is a fascinating branch of chemistry that explores the relationship between electricity and chemical reactions. It delves into the study of how electrons are transferred between molecules and how those electron transfers can be harnessed to drive chemical transformations. This field of study has profound implications in various industries, including energy storage, materials science, and environmental remediation.
In this article, we will delve into 16 surprising facts about electrochemistry that will not only intrigue chemistry enthusiasts but also shed light on the importance and applications of this discipline. From the discovery of electrolysis to the development of cutting-edge electrochemical technologies, these facts showcase the significant role electrochemistry plays in shaping the world we live in.
Key Takeaways:
- Electrochemistry is all about the cool ways electricity and chemistry team up, like making batteries and fighting corrosion. It’s like a superpower for making our world better!
- Electrochemistry isn’t just for scientists—it’s in our everyday lives, from the batteries in our toys to the way our muscles move. It’s like a secret ingredient in making things work!
Electrochemistry is the study of chemical processes that involve electric currents.
Electrochemistry is a branch of chemistry that deals with the interaction between electricity and chemical reactions. It explores how electrical energy can be used to drive chemical reactions or how chemical reactions can generate electricity. This field plays a crucial role in various disciplines such as energy storage, corrosion prevention, electroplating, and even the functioning of our own cells.
Alessandro Volta invented the first battery, known as the Voltaic Pile, in 1800.
Italian physicist Alessandro Volta is credited with the invention of the first battery, which consisted of alternating copper and zinc discs stacked on top of each other. This groundbreaking invention paved the way for the development of modern batteries and the widespread use of electrochemical cells.
Electroplating is a widely used electrochemical process.
Electroplating involves depositing a thin layer of metal onto an object through an electrochemical cell. This process is commonly used for creating decorative or protective coatings on various surfaces, such as jewelry, automotive parts, and even electronic components.
Electrolysis is a key process in electrochemistry.
Electrolysis involves using an electric current to drive a non-spontaneous chemical reaction. Through electrolysis, compounds can be separated into their constituent elements or complex molecules can be synthesized. This process is crucial in industries such as metal extraction, water purification, and the production of chemicals and fuels.
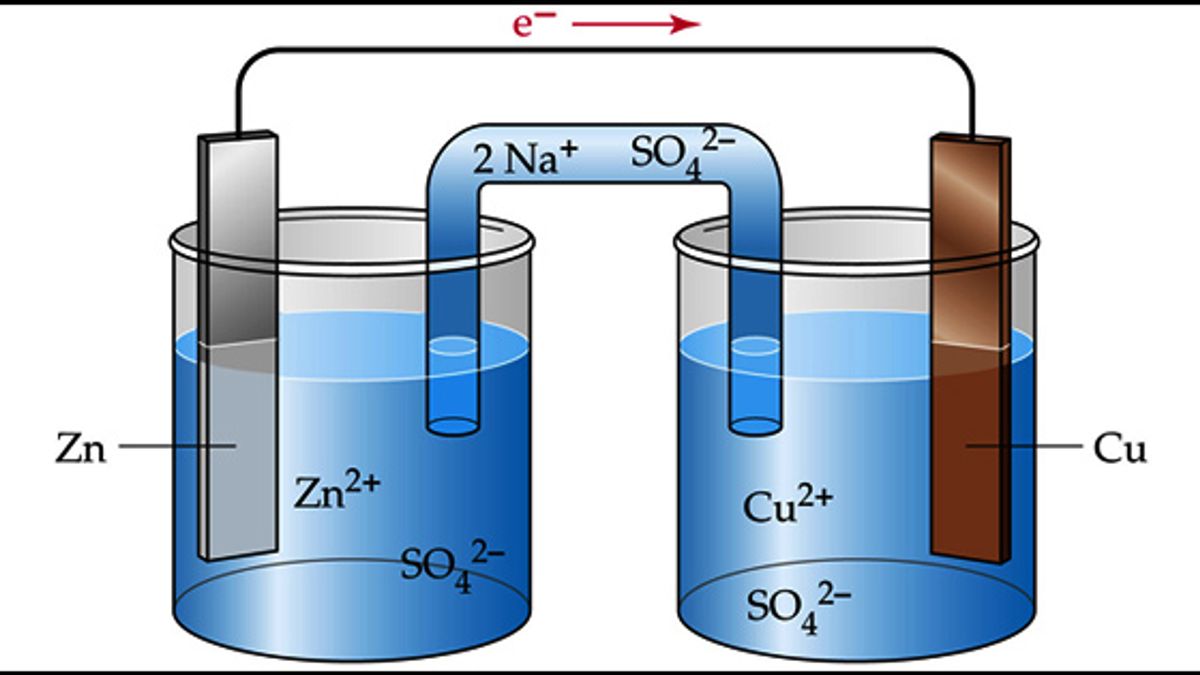
Electrochemical cells are classified into two main types: galvanic cells and electrolytic cells.
Galvanic cells, also known as voltaic cells, convert chemical energy into electrical energy, producing a spontaneous flow of electrons. On the other hand, electrolytic cells use electrical energy to drive a non-spontaneous chemical reaction, requiring an external power source.
The electrolyte in an electrochemical cell serves as a medium for ion migration.
An electrolyte is a substance that can conduct electricity through the movement of ions. It fills the space between the two electrodes in an electrochemical cell and facilitates the flow of ions, allowing the transfer of charge between them.
Batteries are portable electrochemical cells that provide an electric current.
Batteries store chemical energy and convert it into electrical energy when needed. They are commonly used in portable electronic devices, vehicles, and as backup power sources. From simple disposable batteries to advanced rechargeable ones, they have revolutionized numerous industries and transformed our daily lives.
Fuel cells are electrochemical devices that convert the energy of a chemical reaction into electrical energy.
Fuel cells are electrochemical devices that produce electricity by combining a fuel and an oxidant. They have the potential to replace traditional combustion engines with more efficient and environmentally friendly power sources. Fuel cells are used in various applications, including transportation, stationary power generation, and portable electronics.
Electrochemical sensors are used for detecting and measuring various substances.
Electrochemical sensors utilize the electrochemical properties of substances to detect and measure their concentrations. They find applications in environmental monitoring, medical diagnostics, food quality control, and many other fields where fast and accurate analysis is required.
The electrode potential is a measure of the tendency of an electrode to gain or lose electrons.
Electrode potential, also known as redox potential, provides information about the reactivity of an electrode in an electrochemical cell. It quantifies the tendency of an electrode to undergo oxidation or reduction reactions, enabling the prediction of the direction and spontaneity of electron flow.
Electrochemical corrosion can lead to the deterioration of metallic structures.
Corrosion is an electrochemical process that occurs when metal reacts with its environment, resulting in the degradation of the metal. It is a major concern in industries and infrastructure, leading to structural failures and economic losses. Various methods, including coatings and sacrificial anodes, are employed to prevent or minimize corrosion.
The Nernst equation relates the electrode potential to the concentration of species involved in the electrochemical reaction.
The Nernst equation, formulated by Walther Nernst, provides a mathematical relationship between the electrode potential, the concentration of species in the electrochemical cell, and other relevant factors. It is widely used in electrochemical analysis and allows for the calculation of equilibrium potentials under different conditions.
Electrochemical cells are used in renewable energy systems, such as solar cells and fuel cells.
Solar cells, also known as photovoltaic cells, convert sunlight into electricity through an electrochemical process called the photovoltaic effect. Similarly, fuel cells utilize electrochemical reactions to produce electricity from hydrogen and oxygen, offering a clean and sustainable energy source for the future.
Electrochemistry plays a crucial role in understanding and developing batteries for energy storage.
Batteries are essential components of energy storage systems, playing a vital role in renewable energy integration, electric vehicles, and grid applications. Advances in electrochemistry have enabled the development of high-performance batteries with improved energy density, longer lifetimes, and faster charging capabilities.
Electrochemical impedance spectroscopy is a powerful technique for studying electrochemical systems.
Electrochemical impedance spectroscopy (EIS) is a non-destructive technique used to analyze the electrical behavior of electrochemical systems. By measuring the impedance at different frequencies, EIS provides valuable insights into reaction kinetics, charge transfer processes, and the performance of electrochemical devices.
Electrochemical processes are integral to biological systems, including nerve signaling and muscle contraction.
In biological systems, electrochemical processes play a vital role in various physiological functions. Nerve signaling, for example, involves the generation and propagation of electrical impulses through the flow of ions. Muscle contraction also relies on electrochemical processes, enabling the communication between nerve cells and muscle fibers.
Conclusion
In conclusion, electrochemistry is a fascinating field that plays a crucial role in our everyday lives. From providing insights into chemical reactions to enabling the development of new technologies, electrochemistry is at the forefront of scientific advancements.
Through this article, we have explored 16 surprising facts about electrochemistry. We have learned about the fundamental principles, applications, and impact of electrochemistry on various industries, such as energy storage, environmental remediation, and medicine.
Understanding electrochemistry can help unlock new possibilities for sustainable energy solutions, advancements in materials science, and innovative medical treatments. By studying and harnessing the power of electrochemistry, scientists continually push the boundaries of what is possible.
As we continue to delve deeper into the world of electrochemistry, there is no doubt that even more exciting discoveries and inventions await us in the future.
FAQs
1. What is electrochemistry?
Electrochemistry is the branch of chemistry that deals with the study of chemical reactions that involve the transfer of electrons between substances.
2. How is electrochemistry used in everyday life?
Electrochemistry has numerous applications in our daily lives, such as in batteries, electroplating, corrosion prevention, water electrolysis, and fuel cells.
3. What are some practical applications of electrochemistry in industry?
Electrochemistry is widely used in various industries for processes like metal extraction, wastewater treatment, chemical synthesis, and electrorefining.
4. What are some emerging fields in electrochemistry?
Some emerging fields in electrochemistry include the development of supercapacitors, electrochemical sensors, and electrochemical conversion technologies for renewable energy.
5. What is the importance of electrochemistry in medicine?
Electrochemistry plays a crucial role in medical devices such as pacemakers, biosensors, and drug delivery systems, enabling advancements in healthcare and personalized medicine.
Electrochemistry holds countless surprises, from the inner workings of batteries to the intricacies of corrosion. Delving deeper into this fascinating field reveals even more astonishing facts. Explore the electrochemical series to understand how metals react in different environments. Faraday's laws of electrolysis shed light on the quantitative aspects of electrochemical reactions, while the components and processes within electrochemical cells showcase the elegance of this branch of science. Continue your journey through the captivating world of electrochemistry by uncovering more remarkable facts in our related articles.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
