Faraday’s Laws of Electrolysis are fundamental principles in the field of electrochemistry. They were first proposed by the renowned British scientist Michael Faraday in the 19th century and have since been considered a cornerstone of our understanding of chemical reactions involving electricity.
In this article, we will delve into the fascinating world of Faraday’s Laws of Electrolysis and explore 15 extraordinary facts about them. From the basic principles to their practical applications, we will uncover the significance of these laws in various industries and scientific advancements.
So, whether you are a chemistry enthusiast, a student, or simply curious about the mysteries of electrochemistry, join us on this journey to discover the amazing insights offered by Faraday’s Laws of Electrolysis.
Key Takeaways:
- Faraday’s Laws of Electrolysis, discovered by Michael Faraday, explain how electricity can control chemical reactions. These laws are crucial for processes like electroplating and understanding power sources’ efficiency.
- Faraday’s Laws have far-reaching impacts, from guiding metal coating processes to inspiring sustainable technologies. They continue to shape our understanding of electricity and chemical reactions, influencing diverse fields of science and technology.
The Laws of Electrolysis were formulated by Michael Faraday.
Michael Faraday, a renowned English scientist, conducted extensive experiments to establish the laws that govern electrolytic reactions. His groundbreaking work laid the foundation for our understanding of electrochemistry.
The First Law of Electrolysis relates to the amount of substance produced.
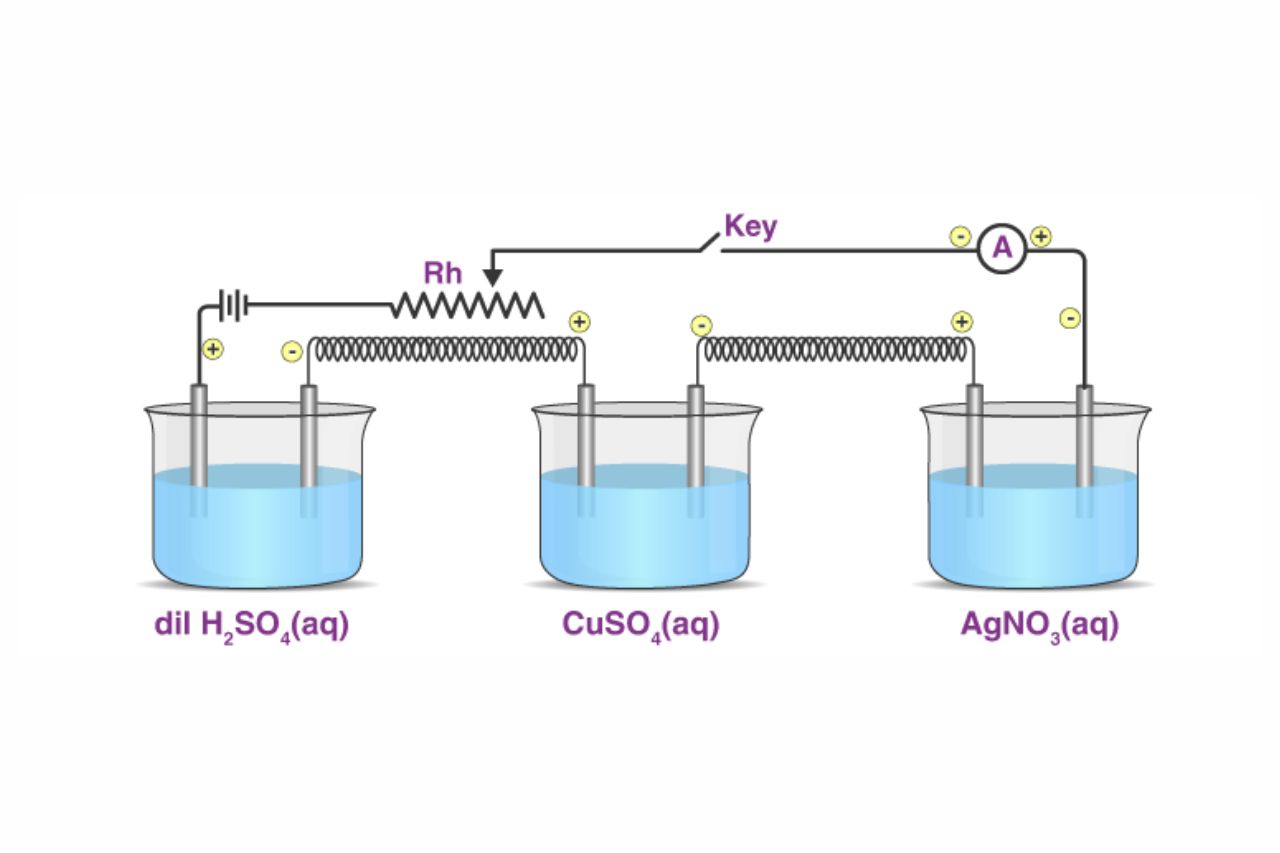
The First Law states that the amount of substance produced or consumed during an electrolytic reaction is directly proportional to the quantity of electricity passed through the solution.
The Second Law of Electrolysis is about the equivalence of different elements.
The Second Law states that the amounts of different substances produced or consumed during electrolysis are directly proportional to their equivalent weights, which are related to their atomic masses.
Faraday’s constant is crucial for calculating the quantity of electricity.
Faraday’s constant, denoted as F, is a key value used to convert the quantity of electricity into the number of moles of substance involved in the electrolytic reaction. It is approximately equal to 96,485.33289 coulombs per mole.
Electrolysis is the process of using electricity to drive a non-spontaneous chemical reaction.
Faraday’s Laws of Electrolysis provide the theoretical framework for understanding and manipulating chemical reactions using electrical energy. Electrolysis finds applications in various fields, including metal refining, electroplating, and the production of industrial chemicals.
Electrolytes are essential for conducting electricity during electrolysis.
In order for an electrolytic reaction to occur, the presence of an electrolyte is necessary. Electrolytes are substances that dissociate into ions when dissolved in a solvent, enabling the flow of electric current through the solution.
Faraday’s Laws hold true for both molten salts and electrolytic solutions.
Whether the electrolyte is in a molten state or dissolved in a solvent, Faraday’s Laws of Electrolysis still apply. This allows for a wide range of applications in both industrial processes and laboratory experiments.
Faraday’s Laws have been validated through experimental observations.
These laws have withstood the test of time and have been repeatedly confirmed by numerous experiments. The accuracy and reliability of Faraday’s Laws make them essential in the field of electrochemistry.
Faraday’s Laws paved the way for the quantitative study of electrochemical reactions.
Prior to Faraday’s groundbreaking work, the field of electrochemistry lacked a systematic approach to quantifying the relationship between electricity and chemical reactions. His laws provided a framework for measuring and understanding these processes.
Faraday’s Laws are integral to the determination of electrode potentials.
The application of Faraday’s Laws allows scientists to determine the standard electrode potentials of different half-cells, which are crucial for predicting the direction and feasibility of redox reactions.
The Laws of Electrolysis are widely used in the field of electroplating.
Electroplating is a process that involves coating a metal surface with a layer of another metal using an electrolytic cell. Faraday’s Laws guide this process by ensuring precise control over the deposition of the plating metal.
Faraday’s Laws provide insight into the efficiency of power sources.
By studying the relationship between electricity consumption and the resulting chemical reactions, scientists can evaluate the efficiency of different power sources, such as batteries and fuel cells.
The concept of Faraday’s Laws extends beyond electrolysis.
The principles established by Faraday have applications in various fields of science and technology, including electrochemical sensors, corrosion studies, and even neurobiology.
The Laws of Electrolysis have societal and environmental implications.
Understanding Faraday’s Laws is crucial for developing sustainable technologies and exploring alternative energy sources. These principles inform the design and optimization of electrochemical processes that can reduce environmental impact.
Faraday’s Laws continue to inspire scientists and researchers today.
The legacy of Michael Faraday and his Laws of Electrolysis lives on. Scientists still work to expand our understanding of electrochemical reactions and develop innovative applications based on these fundamental principles.
In conclusion, Faraday’s Laws of Electrolysis are not only extraordinary but also foundational in the field of electrochemistry. With their wide-ranging applications and impact on various disciplines, these laws continue to shape our understanding of the relationship between electricity and chemical reactions.
Conclusion
In conclusion, Faraday’s Laws of Electrolysis are fundamental principles in chemistry that describe the relationship between the amount of substance produced or consumed during an electrochemical reaction and the amount of electricity passed through the system. These laws were developed by the renowned English scientist Michael Faraday in the early 19th century and have since played a crucial role in understanding and harnessing the power of electrochemistry.
Faraday’s First Law states that the amount of substance liberated or deposited at an electrode is directly proportional to the quantity of electricity passed through the electrolyte solution. This law provides the foundation for quantitative analysis and allows scientists to calculate the stoichiometry of reactions taking place in electrolytic cells.
Faraday’s Second Law states that the amounts of different substances liberated or deposited by the same quantity of electricity are directly proportional to their respective equivalent masses. This law helps in determining the relative reactivity and equivalent weights of different elements and compounds.
By grasping the concepts behind Faraday’s Laws of Electrolysis, we can understand the fundamental principles of electrochemistry and its applications in various fields such as energy storage, electroplating, and industrial processes. These laws continue to be relevant and serve as the basis for further advancements in the field of electrochemistry.
FAQs
Q: Who discovered Faraday’s Laws of Electrolysis?
A: Faraday’s Laws of Electrolysis were discovered by the English scientist Michael Faraday.
Q: What do Faraday’s Laws of Electrolysis describe?
A: Faraday’s Laws of Electrolysis describe the relationship between the amount of substance produced or consumed during an electrochemical reaction and the amount of electricity passed through the system.
Q: What is Faraday’s First Law?
A: Faraday’s First Law states that the amount of substance liberated or deposited at an electrode is directly proportional to the quantity of electricity passed through the electrolyte solution.
Q: What is Faraday’s Second Law?
A: Faraday’s Second Law states that the amounts of different substances liberated or deposited by the same quantity of electricity are directly proportional to their respective equivalent masses.
Q: What are the applications of Faraday’s Laws of Electrolysis?
A: Faraday’s Laws of Electrolysis have various applications in fields such as energy storage, electroplating, and industrial processes.
Faraday's groundbreaking work in electrolysis continues to captivate scientists and curious minds alike. His laws provide a solid foundation for understanding the intricacies of electrochemical reactions. If you found these facts intriguing, you might also enjoy exploring the fascinating world of Faraday's Law of Electrolysis in greater depth. Unraveling the mysteries behind this fundamental principle will surely spark your curiosity and leave you eager to learn more about the remarkable contributions of Michael Faraday to the field of electrochemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
