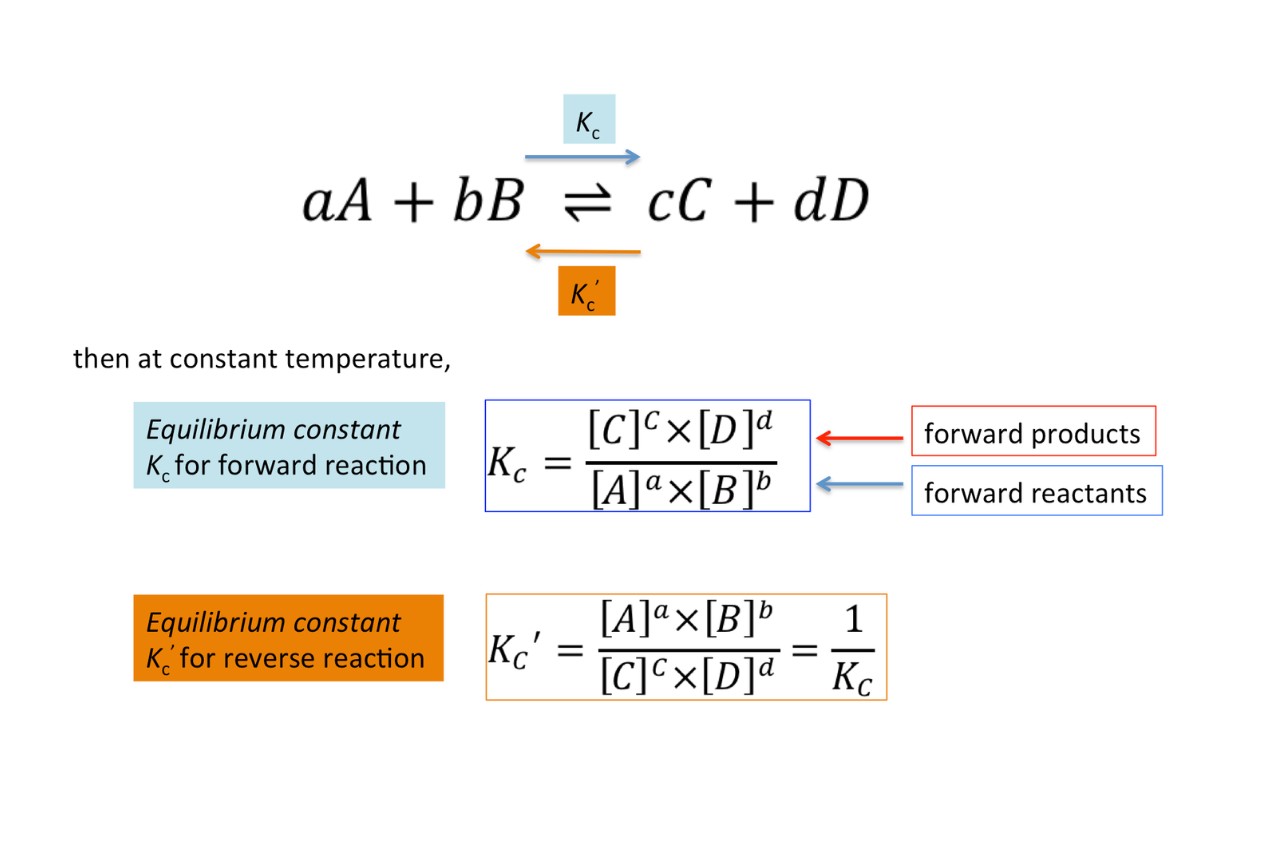
Equilibrium constant, often represented as Kc, is a fundamental concept in chemistry that plays a crucial role in understanding chemical reactions. It provides valuable information about the balance between reactants and products in a chemical system at equilibrium. In simple terms, Kc indicates the extent to which a reaction proceeds in both the forward and reverse directions.
Unlocking the mysteries of equilibrium constant can help us gain deeper insights into the behavior of different chemical species. Whether you are a chemistry enthusiast or a student learning the principles of chemical equilibrium, understanding these fascinating facts about Kc can enhance your knowledge and appreciation for this important concept.
Key Takeaways:
- Equilibrium Constant (Kc) measures the extent of a chemical reaction at a specific temperature, showing if it favors products or reactants. It’s not affected by initial concentrations and is temperature-specific.
- Kc is determined by the Law of Mass Action and can be used to compare the reaction quotient (Qc) to determine if a reaction has reached equilibrium. It’s also specific to the temperature at which the equilibrium is established.
What is the Equilibrium Constant (Kc)?
The Equilibrium Constant (Kc) is a quantitative measurement used in chemical reactions to determine the concentration of reactants and products at equilibrium. It represents the ratio of product concentrations to reactant concentrations at a specific temperature.
Kc Is Dependent on Temperature
The value of Kc is influenced by changes in temperature. An increase in temperature can shift the equilibrium position, resulting in a change in the equilibrium constant.
The Value of Kc Indicates the Extent of the Reaction
A high value of Kc indicates that the equilibrium lies towards the products, suggesting that the reaction is more favorable and proceeds to a greater extent. Conversely, a low value of Kc indicates that the equilibrium favors the reactants.
Kc Is Not Affected by Initial Concentrations
The equilibrium constant, Kc, remains the same regardless of the initial concentrations of the reactants and products. It is solely dependent on the temperature and the stoichiometry of the balanced chemical equation.
Kc Can Be Determined from the Balanced Chemical Equation
The equilibrium constant can be calculated using the concentrations of the reactants and products at equilibrium, as determined by the stoichiometric coefficients in the balanced chemical equation.
Kc and the Law of Mass Action
Kc is directly related to the Law of Mass Action, which states that the rate of a chemical reaction is directly proportional to the product of the concentrations of the reactants raised to the power of their respective stoichiometric coefficients.
Kc for Reversible Reactions
In reversible reactions, where reactants can form products and products can also react to reform reactants, Kc expresses the ratio of product-to-reactant concentrations at equilibrium.
Units of Kc
The units of Kc depend on the overall order of the reaction. If the reaction is first order, Kc has units of moles per liter (mol/L). For second-order reactions, the units are (mol/L)^2, and so on.
Kc and Reaction Quotient (Qc)
Kc is often compared to the reaction quotient (Qc), which is calculated using the same formula but based on concentrations that are not necessarily at equilibrium. By comparing Kc and Qc, one can determine whether a reaction has reached equilibrium or is still proceeding.
Changing Pressure Does Not Affect Kc
The equilibrium constant, Kc, is independent of changes in pressure as long as the number of moles of gas does not change. This is due to the fact that equilibrium expressions are based on the concentrations of substances, not their pressures.
Kc and Reaction Rates
Kc does not provide any information about the speed or rate at which a reaction occurs. The equilibrium constant only describes the concentrations of the reactants and products at equilibrium.
Kc Is Temperature-Specific
The value of Kc for a particular reaction is specific to the temperature at which the equilibrium is established. Changing the temperature can result in a different equilibrium constant value.
Kc and Le Chatelier’s Principle
According to Le Chatelier’s Principle, an increase in temperature will shift the equilibrium in the direction that absorbs heat, while a decrease in temperature will shift the equilibrium in the direction that releases heat. These temperature changes can affect the value of Kc.
Conclusion
In conclusion, the equilibrium constant (Kc) is a fundamental concept in chemistry that plays a crucial role in understanding the behavior of chemical reactions. Through the equilibrium constant, scientists can determine the extent to which a reaction proceeds and predict the concentrations of reactants and products at equilibrium.During the study of equilibrium constant, you may have come across several fascinating facts that shed light on its importance and implications. From the significance of different values of Kc to the factors influencing its magnitude, understanding these facts can deepen your knowledge and appreciation for this concept.Equilibrium constant is not only relevant in the field of chemistry but also has practical applications in various industries. It provides insights into reaction kinetics, helps in designing optimal reaction conditions, and aids in the development of efficient chemical processes.By delving into the fascinating world of equilibrium constant, you can unlock a deeper understanding of chemical reactions, equilibria, and the principles that govern them. Whether you are a budding chemist or a curious learner, exploring the intricacies of Kc can broaden your scientific horizons and open doors to countless possibilities.
FAQs
1. What does the equilibrium constant (Kc) signify?
The equilibrium constant, represented by Kc, indicates the ratio of the concentrations of products to the concentrations of reactants in a chemical reaction at equilibrium. It provides a quantitative measure of the extent to which a reaction proceeds towards the formation of products.
2. Can the value of Kc change?
No, the value of Kc is constant at a given temperature and does not change unless the temperature is altered. However, the concentrations of reactants and products can change, leading to a shift in the equilibrium position and a new value of Kc.
3. What do different values of Kc signify?
If the value of Kc is greater than 1, it indicates that the concentration of products is higher than the concentration of reactants at equilibrium, favoring the formation of products. Conversely, if the value of Kc is less than 1, it suggests that the concentration of reactants is higher, favoring the reverse reaction.
4. Can Kc determine the rate of a reaction?
No, the equilibrium constant (Kc) does not provide information about the rate at which a reaction occurs. It only gives insights into the relative concentrations of reactants and products at equilibrium.
5. What factors can affect the magnitude of Kc?
The equilibrium constant is influenced by factors such as temperature, pressure, and the presence of catalysts. Changes in these conditions can lead to a shift in the equilibrium position, resulting in a different value of Kc.
6. Can Kc have a negative value?
No, the equilibrium constant (Kc) cannot have a negative value. It is always a positive number or zero, indicating the ratio of products to reactants at equilibrium.
7. What is the significance of Kc in chemical industries?
Equilibrium constant plays a crucial role in chemical industries by providing insights into reaction kinetics, optimizing reaction conditions, and designing efficient chemical processes. It helps in maximizing product yields and improving the overall efficiency of chemical reactions.
8. Can Kc be used to predict the feasibility of a reaction?
Yes, the magnitude of the equilibrium constant (Kc) can indicate the feasibility of a reaction. If the value of Kc is very large, it suggests that the reaction strongly favors the formation of products. On the other hand, a small value of Kc indicates that the reaction is less likely to proceed towards product formation.
9. How can we calculate Kc?
Kc can be determined by experimentally measuring the concentrations of reactants and products at equilibrium and applying the appropriate formula based on the balanced chemical equation. The concentrations can be determined using techniques such as spectroscopy or chromatography.
10. Can Kc be used to compare different reactions?
Yes, the equilibrium constant (Kc) can be used to compare the relative extents of different reactions. By comparing the magnitudes of Kc for different reactions, it is possible to determine which reaction proceeds farther towards product formation.
Exploring equilibrium constants is just the beginning of your journey through chemistry's fascinating concepts. Dive deeper into the world of reaction quotients, where you'll discover how they relate to chemical reactions and their progress. For a broader perspective, uncover the captivating realm of physical chemistry, which encompasses thermodynamics, kinetics, and quantum mechanics. And if you're curious about how systems achieve balance, investigate the intriguing principles of chemical equilibrium, where forward and reverse reactions reach a state of dynamic harmony. Keep learning, and you'll soon master the fundamental pillars of chemistry!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
