Are you fascinated by the world of chemistry? Do you love delving into the intricacies of atoms and molecules? If so, you’ve likely come across the concept of Lewis dot structure during your studies. Lewis dot structure is a visual representation of the valence electrons of an atom or molecule, allowing us to understand and predict their chemical behavior. In this article, we will explore 14 intriguing facts about Lewis dot structures that will deepen your understanding and appreciation for their significance in the world of chemistry. From the basics of electron configuration to the complexities of resonance structures, get ready to uncover the secrets behind these captivating diagrams. So, let’s dive in and explore the fascinating realm of Lewis dot structures!
Key Takeaways:
- Lewis Dot Structure is a cool way to see how atoms bond in molecules using dots and lines. It helps us understand how atoms share electrons to form chemical bonds.
- By using Lewis Dot Structure, we can predict the shape of molecules and understand how they react with each other. It’s like a secret code to unlock the mysteries of chemical bonding!
Lewis Dot Structure helps visualize the bonding in molecules.
The Lewis Dot Structure is a diagrammatic representation of the chemical bonding in a molecule. It helps us understand how atoms arrange themselves and share electrons to form chemical bonds.
It was developed by American chemist Gilbert N. Lewis in the early 20th century.
Gilbert N. Lewis introduced the concept of Lewis Dot Structure in 1916 as a way to simplify the understanding of chemical bonding. His groundbreaking work has had a significant impact on the field of chemistry.
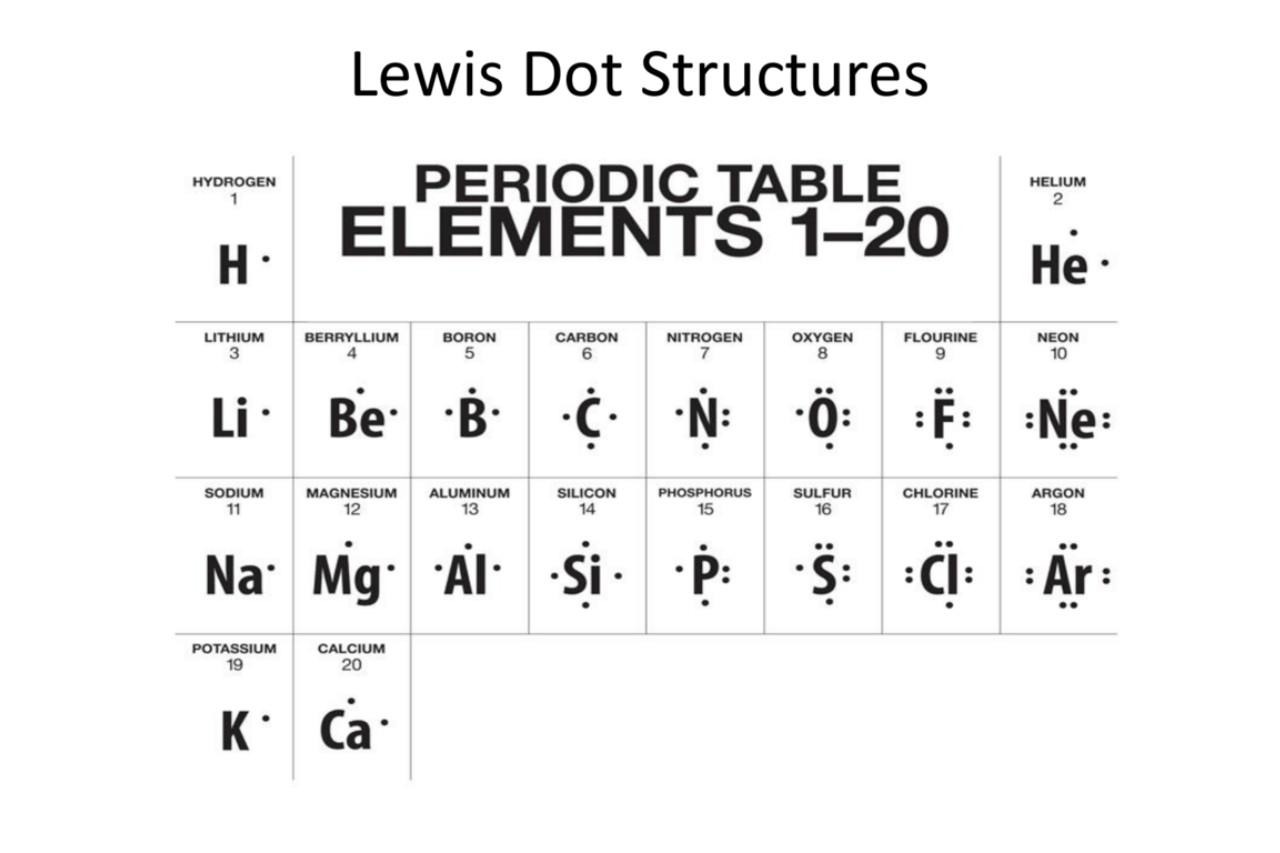
The structure uses dots to represent valence electrons.
In a Lewis Dot Structure, the valence electrons of an atom are represented by dots placed around the atomic symbol. This notation allows us to easily determine the number of valence electrons an atom possesses.
It follows the octet rule.
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration of eight valence electrons. Lewis Dot Structure helps us determine how atoms achieve this octet configuration.
The central atom is represented by its atomic symbol.
In a Lewis Dot Structure of a molecule, the central atom is represented by its atomic symbol, surrounded by the dots representing its valence electrons. The other atoms are then connected to the central atom using lines to represent the bonds.
Non-bonding pairs of electrons are represented by pairs of dots.
In addition to bonding pairs, Lewis Dot Structure also includes non-bonding pairs of electrons. These pairs are represented by pairs of dots placed next to the atomic symbol of the atom.
Double and triple bonds are represented by multiple lines.
In cases where atoms share more than one pair of electrons, double or triple bonds are formed. In Lewis Dot Structure, these multiple bonds are represented by multiple lines between the atoms.
It helps predict molecular geometry.
By understanding the Lewis Dot Structure of a molecule, we can predict its three-dimensional shape or molecular geometry. This knowledge is crucial in determining the molecule’s properties and behavior.
Lewis Dot Structure aids in understanding chemical reactions.
With the help of Lewis Dot Structure, chemists can analyze and predict how different atoms and molecules react with each other. It provides valuable insights into the formation and breaking of chemical bonds.
It is widely used in introductory chemistry courses.
The Lewis Dot Structure is an essential tool in teaching and learning chemistry, particularly at the introductory level. It simplifies the understanding of chemical bonding and helps students visualize molecular structures.
Lewis Dot Structure can be used for both organic and inorganic molecules.
Whether it’s a simple organic compound or a complex inorganic molecule, Lewis Dot Structure can be applied to represent their bonding patterns and valence electron arrangements.
It provides a foundation for understanding molecular polarity.
By examining the distribution of electrons in a molecule’s Lewis Dot Structure, we can determine if the molecule is polar or nonpolar. This knowledge helps us understand various physical and chemical properties of substances.
Lewis Dot Structure can be expanded to include resonance structures.
In cases where a molecule can have multiple valid Lewis structures, the concept of resonance is used. Resonance structures are different representations of a molecule, and they contribute to its overall stability and reactivity.
Lewis Dot Structure helps in drawing electron-dot diagrams.
Electron-dot diagrams, also known as Lewis Dot Diagrams, are a simplified way of representing the valence electron arrangement of atoms and molecules. Lewis Dot Structure provides the foundation for drawing accurate electron-dot diagrams.
Conclusion
In conclusion, Lewis dot structures are a fundamental concept in chemistry that is used to represent the electrons in an atom’s outermost energy level. They provide valuable information about the bonding and structure of molecules, allowing chemists to predict the properties and reactivity of different compounds.Throughout this article, we have explored 14 intriguing facts about Lewis dot structures. We have learned how to draw and interpret these structures, as well as their significance in determining molecular shapes and bond types. Additionally, we have discovered their applications in various areas of chemistry, such as organic chemistry and biochemistry.Whether you are a student learning the basics of chemistry or a seasoned professional in the field, understanding Lewis dot structures is essential for grasping the fundamental principles of chemical bonding. By mastering this concept, you will be equipped with a powerful tool that will enable you to analyze and predict the behavior of molecules with accuracy.So, continue your exploration of the fascinating world of chemistry, and let Lewis dot structures be your guide to unlocking the secrets of the elements and compounds that surround us.
FAQs
1. What is a Lewis dot structure?
A Lewis dot structure is a representation of the valence electrons in an atom or molecule. It uses dots or crosses to represent electrons and dashes to represent bonds.
2. Why are Lewis dot structures important?
Lewis dot structures are important because they provide a visual representation of how electrons are distributed in a molecule, which helps in understanding its bonding and reactivity.
3. How do you draw a Lewis dot structure?
To draw a Lewis dot structure, count the total number of valence electrons, distribute them as dots around the symbol for each atom, and connect the atoms with single, double, or triple bonds based on the number of valence electrons available.
4. What does a lone pair represent in a Lewis dot structure?
A lone pair represents a pair of electrons that is not involved in bonding with other atoms. They are localized on a single atom and can affect the shape and reactivity of a molecule.
5. Are Lewis dot structures the same as electron dot structures?
Yes, Lewis dot structures and electron dot structures refer to the same concept, which is the representation of valence electrons using dots or crosses.
6. Can Lewis dot structures be used for all molecules?
Lewis dot structures are applicable to most covalent molecules and some ionic compounds. They are primarily used for understanding the bonding and electron distribution in molecules.
7. How do Lewis dot structures help in understanding molecular shapes?
By counting the number of electron groups (lone pairs and bonded atoms) around a central atom, Lewis dot structures can provide insights into the molecular geometry and the presence of any bond angles or distortions.
8. Can Lewis dot structures predict chemical reactions?
While Lewis dot structures alone cannot predict specific reactions, they do provide a foundation for understanding the reactivity of molecules. By analyzing electron distribution and bond types, chemists can make predictions about the potential reactions a compound may undergo.
Mastering Lewis dot structure opens doors to understanding molecular geometry, polarity, and chemical reactions. Grasping this fundamental concept lays the groundwork for exploring more advanced topics in chemistry. For those eager to deepen their knowledge, our article on Lewis structures offers 12 intriguing facts that build upon the basics covered here. Whether you're a budding chemist or simply curious about the world around you, continue your journey of discovery by diving into these related topics.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
