When it comes to the fascinating world of chemistry, there are numerous concepts and principles that capture our attention. One such concept is pOH, which plays a crucial role in understanding the properties of solutions and acids and bases. Derived from the negative logarithm of hydroxide ion concentration, pOH provides valuable insights into the alkalinity of a solution.
In this article, we will explore 12 astounding facts about pOH that will deepen your understanding of this important concept. From its relationship with pH to its significance in chemical reactions, we will delve into the various facets of pOH and how it influences our understanding of chemistry. So, let’s embark on this educational journey and uncover the intriguing world of pOH.
Key Takeaways:
- pOH measures how basic a solution is and is related to pH. It’s crucial in chemistry, biology, and industry, and even affects the environment and our health.
- Knowing pOH helps us understand the strength of bases, water quality, and even how our body maintains its pH balance. It’s a key factor in many aspects of our lives.
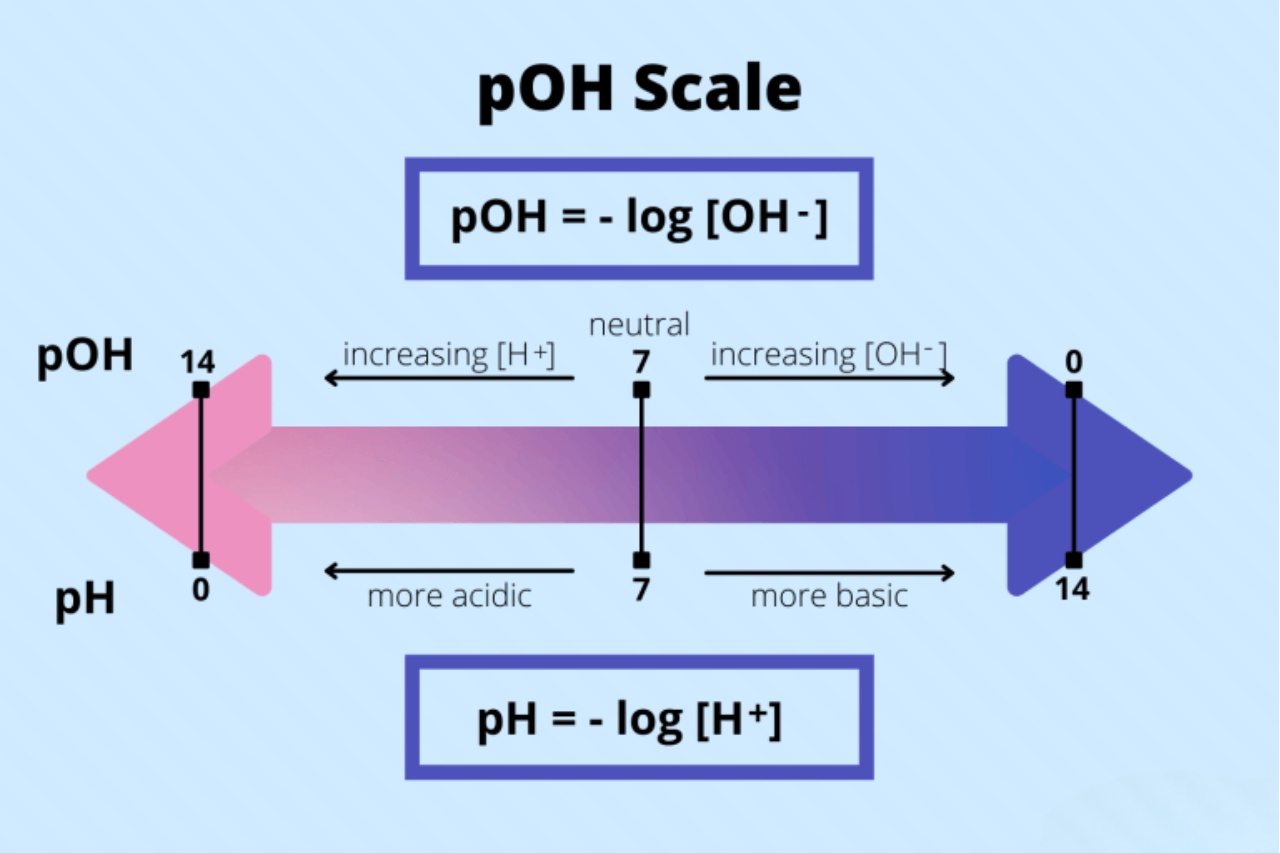
The Definition of pOH
pOH is a measure of the alkalinity or basicity of a solution. It is the negative logarithm of the hydroxide ion concentration in a solution.
pOH and pH Relationship
pOH and pH are related to each other through the equation pOH + pH = This means that if the pOH of a solution is known, its pH can be easily calculated.
Calculation of pOH
To calculate the pOH of a solution, take the negative logarithm of the hydroxide ion concentration. The formula is pOH = -log[OH-].
Interconversion of pOH and OH- Concentration
The OH- concentration can be determined from the pOH by taking the antilog of the negative pOH value. Conversely, the pOH can be calculated from the OH- concentration by taking the negative logarithm of the concentration.
pOH and Acid-Base Reactions
pOH is used to measure the acidity or basicity of a solution in acid-base reactions. It determines the strength of a base, with lower pOH values indicating stronger bases.
pOH of Pure Water
The pOH of pure water at 25°C is 7, which indicates that pure water is neutral. This is because the concentration of hydroxide ions and hydronium ions in pure water is equal.
pOH and Household Items
pOH can be found in various household items such as cleaning products, soaps, and bleach. These products often have high pOH values due to their alkaline nature.
pOH and Human Body
The human body maintains a delicate balance of pH levels to ensure proper functioning. pOH plays a role in regulating the pH of bodily fluids and maintaining overall health.
pOH and Environmental Impact
pOH levels can have an impact on the environment, especially in aquatic systems. Changes in pOH can affect the health of aquatic organisms and disrupt ecosystems.
pOH and Industrial Processes
In industrial processes such as wastewater treatment and chemical manufacturing, monitoring pOH levels is crucial to optimize efficiency and ensure the desired reaction outcomes.
pOH and Water Quality
pOH measurements are used in assessing water quality, particularly in determining the alkalinity of water sources. This information is valuable for maintaining healthy aquatic habitats and drinking water standards.
pOH and Acid-Base Titration
pOH is essential in acid-base titration experiments, where it helps determine the equivalence point of the reaction and the concentration of an unknown acid or base.
Overall, pOH is a significant parameter in understanding the acidity and basicity of solutions. It plays a vital role in various fields, ranging from chemistry and biology to industry and environmental sciences. By knowing these 12 astounding facts about pOH, we can grasp the fundamental concepts and applications of this important chemical measurement.
Conclusion
Understanding the concept of pOH is essential for anyone studying chemistry. By knowing the pOH value, you can determine the concentration of hydroxide ions in a solution and analyze its acidity or basicity.
In this article, we have discussed 12 astounding facts about pOH, including its definition, relationship with pH, calculation, and significance in chemical reactions. We have explored how to convert between pOH and OH- concentration, as well as the role of pOH in acid-base equilibrium.
To summarize, pOH provides valuable insights into the alkalinity of a solution and allows chemists to analyze and manipulate chemical reactions effectively. By understanding these fascinating facts about pOH, you are one step closer to becoming a proficient chemist!
FAQs
Q: What does pOH stand for?
A: pOH stands for the negative logarithm of the hydroxide ion concentration in a solution.
Q: How is pOH related to pH?
A: The relationship between pOH and pH is inverse. The sum of pOH and pH values always equals 14. If the pOH is high, the pH value is low, indicating a more acidic solution, and vice versa.
Q: How do you calculate pOH?
A: To calculate pOH, you take the negative logarithm of the hydroxide ion concentration. The formula is pOH = -log[OH-].
Q: Can pOH be higher than 14?
A: No, pOH cannot be higher than 14 as it represents the alkalinity of a solution. A pOH value of 14 indicates a very acidic solution.
Q: What is the significance of pOH in chemical reactions?
A: pOH helps determine the basicity or acidity of a solution, which is crucial in understanding and manipulating chemical reactions. It also plays a role in acid-base equilibrium calculations and quantitative analysis.
After exploring pOH's fascinating world, why not brighten your home with a Himalayan salt lamp's warm glow? These natural wonders purify air, boost mood, and create a soothing ambiance. Harnessing salt's ancient power, salt lamps make enchanting additions to any space. Discover their benefits today!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
