Acids and bases are fundamental concepts in chemistry, playing a crucial role in various chemical reactions and everyday life. Understanding the properties and behaviors of acids and bases can provide valuable insights into a wide range of phenomena, from the sour taste of citrus fruits to the cleaning power of household substances. In this article, we'll explore 11 fascinating facts about acids and bases, delving into their unique characteristics, real-world applications, and the ways in which they shape the world around us. Whether you're a chemistry enthusiast or simply curious about the science behind these essential substances, join us on a journey through the intriguing realm of acids and bases. So, let's dive in and uncover the captivating world of these chemical compounds!
Key Takeaways:
- Acids and bases are all around us, from the sour taste of lemons to the soapy feel of cleaning products. They can neutralize each other and are crucial in everyday items like soap and food preservation.
- The pH scale measures acids and bases, with 7 being neutral. They play a big role in our bodies, like in our stomachs for digestion, and are essential for chemists to conduct experiments and develop new materials.
Acids and bases are everywhere in our daily lives.
From the food we eat to the products we use, acids and bases play a crucial role in various chemical reactions and processes.
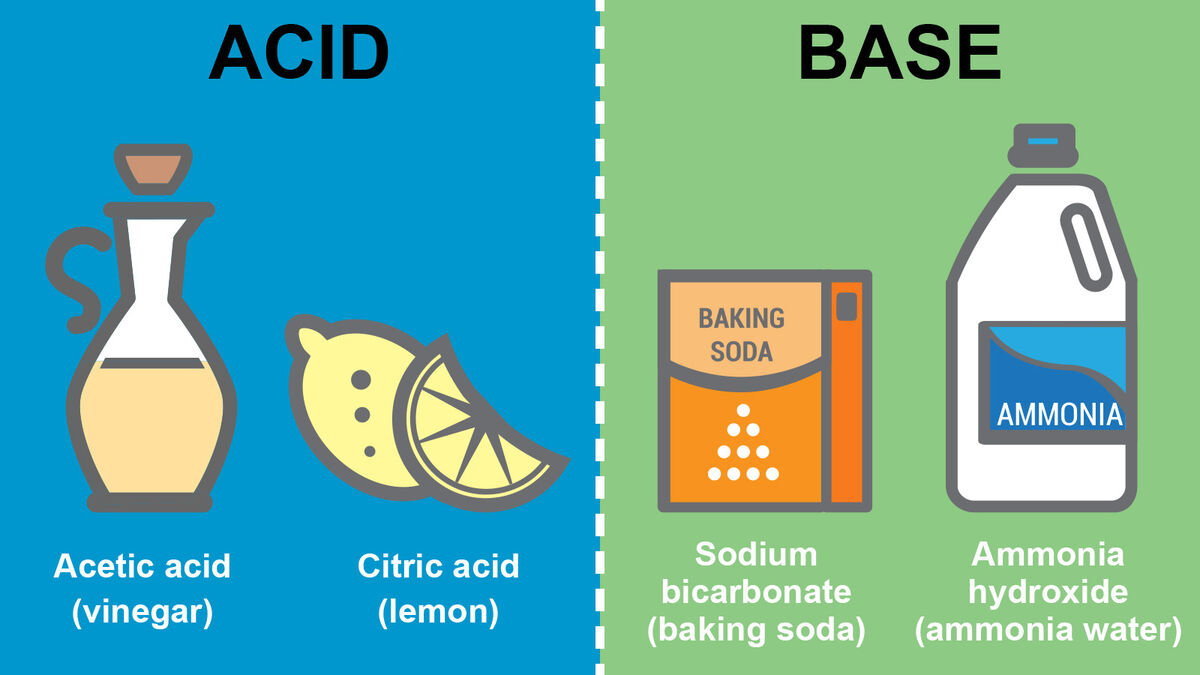
Acids can be found in many sour-tasting foods.
When you bite into a lemon or enjoy a tangy vinegar dressing, you are experiencing the sour taste of acids.
Bases can feel slippery or soapy to the touch.
Have you ever noticed that soapy sensation when using certain cleaning products? That’s the characteristic feel of bases.
Acids and bases are measured on the pH scale.
The pH scale ranges from 0 to 14, with 7 being neutral. Substances with a pH less than 7 are acidic, while those with a pH greater than 7 are basic.
Lemon juice and gastric acid are both acidic.
Our stomachs produce gastric acid to aid in digestion, while the tartness of lemon juice is due to its acidic nature.
Ammonia and bleach are examples of common household bases.
These substances are often used for cleaning and disinfecting due to their basic properties.
Acids and bases can neutralize each other.
When an acid and a base are combined, they undergo a chemical reaction that results in the formation of water and a salt.
Antacids help neutralize excess stomach acid.
Individuals experiencing heartburn or indigestion often rely on antacids to counteract the effects of an overly acidic stomach.
Bases are important in the production of various everyday items.
From soap and paper to textiles and dyes, bases are essential in the manufacturing of numerous household products.
Acids are commonly used in the preservation of food.
Pickling, a process that involves using acidic solutions, is a popular method for preserving fruits and vegetables.
Acids and bases are integral to the field of chemistry.
Chemists rely on their understanding of acids and bases to conduct experiments, develop new materials, and advance scientific knowledge.
Conclusion
Acids and bases play crucial roles in our daily lives, from the foods we eat to the products we use. Understanding their properties and behaviors not only enriches our knowledge of chemistry but also helps us appreciate the world around us. Whether it’s the sour taste of lemon juice or the fizz of a carbonated beverage, the impact of acids and bases is undeniable. Exploring the fun facts about acids and bases allows us to appreciate the fascinating characteristics of these substances and their significance in various applications. By delving into their unique properties and diverse uses, we gain a deeper understanding of the fundamental principles that govern the natural world.
FAQs
What are some common examples of acids and bases in everyday life?
Acids: Lemon juice, vinegar, and soda. Bases: Soap, baking soda, and antacids.
How do acids and bases affect the taste of food?
Acids give foods a sour or tart taste, while bases can impart a bitter or soapy flavor.
What are the potential dangers of acids and bases?
Acids can cause burns and corrosion, while strong bases can also be corrosive and harmful if not handled properly.
How are acids and bases used in industry and manufacturing?
Acids are used in the production of fertilizers, cleaning products, and dyes, while bases are utilized in the manufacture of soaps, paper, and certain types of plastics.
What are the environmental impacts of acids and bases?
Acid rain, caused by sulfuric and nitric acids, can harm ecosystems, while the disposal of strong bases can have detrimental effects on aquatic life.
Hungry for more captivating chemistry knowledge? Quench your thirst by exploring even more intriguing facts about acids and bases. From everyday encounters to groundbreaking scientific theories, there's always something new to learn. Satisfy your curiosity with a deeper look at the pH scale and its significance. Prepare to be amazed as you continue your journey through the world of acids and bases!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
