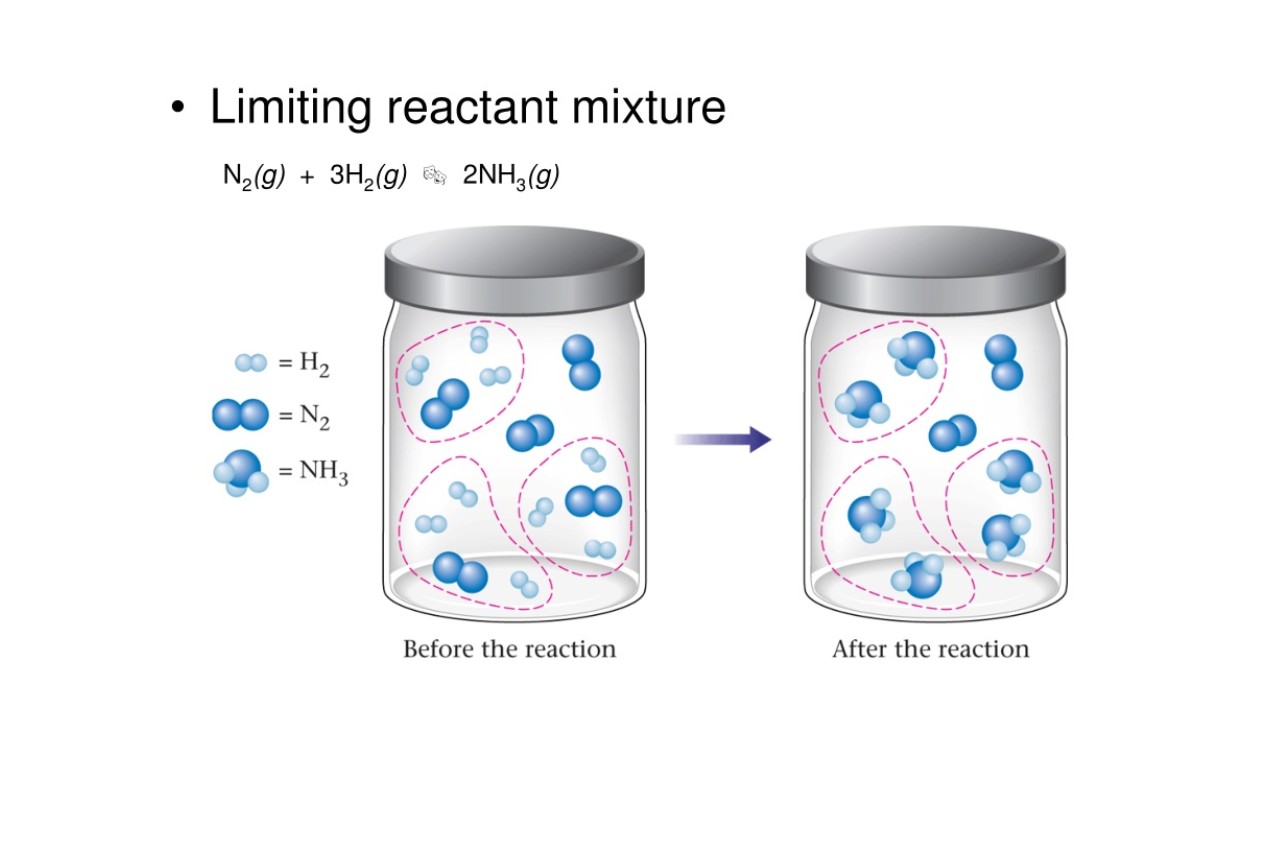
When it comes to chemical reactions, one key concept that plays a crucial role is the limiting reactant. Also known as the limiting reagent, this fascinating concept in chemistry determines the amount of product that can be formed during a reaction. Understanding the concept of limiting reactant is crucial for achieving high yields and efficient use of resources in chemical reactions.
In this article, we will delve into the captivating world of limiting reactants and explore nine astounding facts that will deepen your knowledge of this fundamental chemical concept. From the significance of stoichiometry to the importance of identifying the limiting reactant, we will unravel the intricacies of this topic and shed light on its practical applications in various industries.
So, let’s embark on a journey through the realm of limiting reactants and discover the intriguing facts that make them an essential aspect of chemical reactions.
Key Takeaways:
- The limiting reactant determines how much product can be made in a chemical reaction. It’s like the boss that decides how much work can get done!
- Knowing the limiting reactant helps chemists make the most product with the least waste, keeping reactions safe and efficient. It’s like finding the perfect recipe for a delicious cake!
Limiting Reactant Defined
In chemical reactions, the limiting reactant refers to the substance that is completely consumed in the reaction, thereby limiting the amount of product that can be formed. It determines the maximum amount of product that can be obtained.
Importance in Yield Calculation
The concept of limiting reactant is vital in determining the theoretical yield of a chemical reaction. By identifying the limiting reactant, chemists can accurately calculate the amount of product that will be obtained.
Excess Reactant Exists
In contrast to the limiting reactant, an excess reactant is the substance that is present in greater quantity than required for the reaction to occur. The excess reactant is not completely consumed and remains after the reaction is complete.
Stoichiometry and Limiting Reactant
Stoichiometry plays a crucial role in identifying the limiting reactant. It allows chemists to compare the mole ratio of reactants to determine which one is present in insufficient quantities and will therefore limit the reaction.
Maximum Product Yield
The limiting reactant governs the maximum amount of product that can be formed in a reaction. No matter how much of the other reactant is available, the amount of product formed will be determined solely by the quantity of the limiting reactant.
Efficiency and Limiting Reactant
The concept of the limiting reactant is closely related to the efficiency of a chemical reaction. By ensuring that the reactants are present in the proper stoichiometric ratio, the reaction can proceed with maximum efficiency and minimal waste.
Reactant Excess and Safety
Having an excess of reactants can potentially lead to safety hazards. Certain reactions may become uncontrollable or produce undesired byproducts if the reactants are not carefully measured and controlled.
Analyzing Reactant Ratios
By analyzing the ratios of the reactants, chemists can determine the limiting reactant and make informed decisions regarding the reaction conditions, such as adjusting the quantities of reactants or choosing alternate reaction pathways.
Real-World Applications
The concept of the limiting reactant is essential in various industries, such as pharmaceuticals, manufacturing, and agriculture. It helps ensure efficient use of resources, accurate product yield calculations, and safer chemical processes.
Conclusion
In conclusion, understanding the concept of limiting reactant is crucial in the study of chemistry. It is essential for determining the maximum amount of product that can be formed in a chemical reaction. By identifying the limiting reactant, chemists can optimize reaction conditions and ensure maximum yield. Additionally, knowing the stoichiometry of the reaction allows for precise measurements and calculations.
Furthermore, the concept of limiting reactant has practical applications in various industries, such as pharmaceuticals, agriculture, and manufacturing. By carefully controlling reactant ratios, researchers can produce desired products efficiently and economically.
Overall, studying limiting reactant not only deepens our understanding of chemical reactions but also empowers us to apply this knowledge in real-world scenarios. It is an essential concept for both students and professionals in the field of chemistry.
FAQs
Q: What is a limiting reactant?
A: A limiting reactant is the substance that is completely consumed in a chemical reaction, limiting the amount of product that can be formed.
Q: How do you determine the limiting reactant?
A: The limiting reactant can be determined by comparing the mole ratio of reactants in the balanced chemical equation with the actual amounts of reactants present in the reaction.
Q: Why is it important to identify the limiting reactant?
A: Identifying the limiting reactant allows chemists to calculate the theoretical yield of a reaction, which helps in optimizing reaction conditions and ensuring maximum product formation.
Q: Can multiple reactants be limiting reactants at the same time?
A: No, only one reactant can be the limiting reactant in a given reaction. The reactant that is present in the least amount determines the maximum amount of product that can be formed.
Q: How does the limiting reactant affect the yield of a reaction?
A: The limiting reactant determines the maximum amount of product that can be formed. Any excess of the other reactant(s) beyond the stoichiometric ratio will not be utilized and will not contribute to the yield of the reaction.
Q: Can the limiting reactant change in different reactions?
A: Yes, the limiting reactant can vary depending on the equation and the amounts of reactants used. It is essential to determine the limiting reactant for each specific reaction.
Q: What is the significance of stoichiometry in determining the limiting reactant?
A: Stoichiometry is the quantitative relationship between reactants and products in a chemical equation. It allows for the calculation of reactant ratios and helps in identifying the limiting reactant.
Q: Are there any practical applications of understanding limiting reactant?
A: Yes, understanding limiting reactant has practical applications in industries such as manufacturing, pharmaceuticals, and agriculture, where precise control of reactant ratios is necessary to achieve desired products efficiently.
Q: Can a catalyst be a limiting reactant?
A: No, a catalyst is not consumed in the reaction. It increases the rate of the reaction by providing an alternative pathway, but it does not participate in the stoichiometry of the reaction.
Limiting reactant plays a crucial role in chemical reactions, determining reaction yields and efficiency. Understanding stoichiometry helps identify limiting reactants, ensuring maximum product yields while maintaining safety through proper reactant ratios. Mastering these concepts is essential for optimizing reactions in various real-world applications. For those curious about the intricacies of chemical reactions, exploring the enigmatic world of stoichiometry can provide valuable insights into the fascinating interplay between reactants and products.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
