Understanding the concept of critical temperature (Tc) is essential in the field of chemistry. This parameter plays a significant role in several chemical processes and applications. Critical temperature is defined as the temperature at which a substance transitions from one phase to another, typically from a gas to a liquid or from a liquid to a solid. It represents the threshold at which the molecular forces of attraction overcome the thermal energy of the substance.
In this article, we will explore eight intriguing facts about critical temperature (Tc) that shed light on its importance and applications. From its relationship with superconductivity to its impact on cryogenic technologies, these facts highlight the role of critical temperature in various scientific and industrial contexts. So, let’s dive in and uncover the fascinating world of critical temperature!
Key Takeaways:
- Critical temperature (Tc) is the highest temperature at which a substance can exist as a liquid, impacting phase transitions and material behavior.
- Understanding Tc is crucial in fields like superconductivity, high-pressure chemistry, and environmental impact of substances like carbon dioxide.
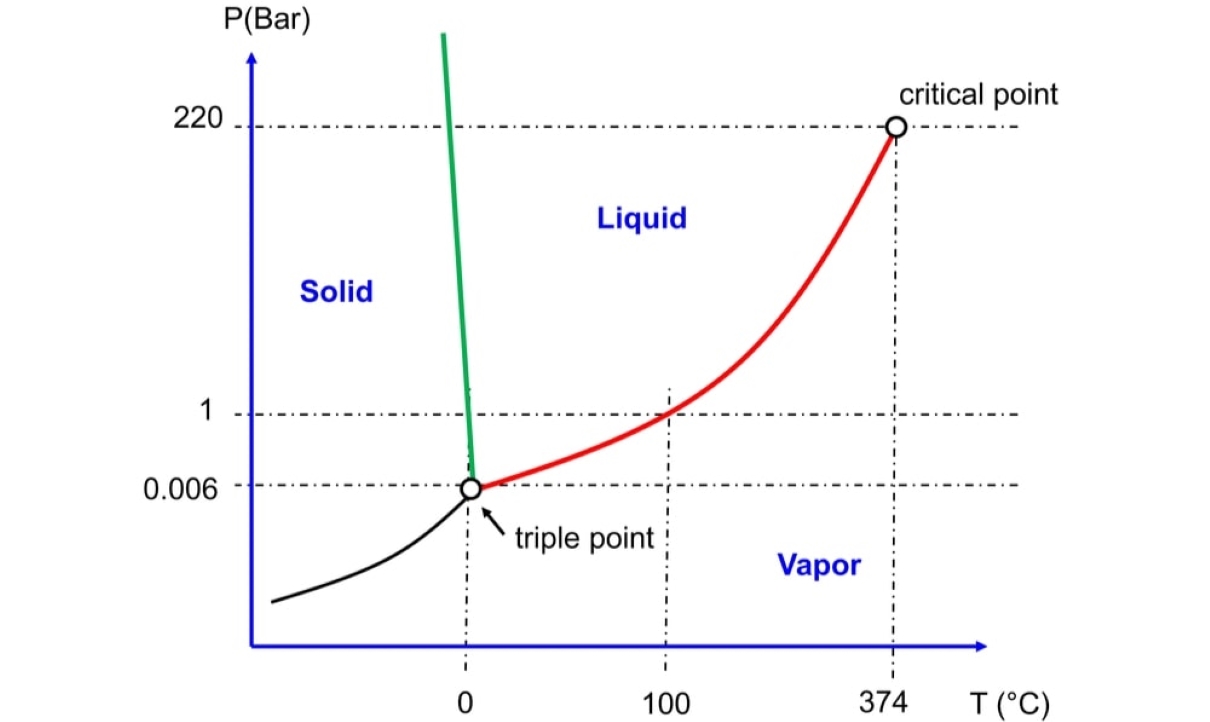
Definition of Critical Temperature (Tc)
The critical temperature (Tc) is the highest temperature at which a substance can exist as a liquid. Above this temperature, it becomes a gas, regardless of the pressure applied. Conversely, below the critical temperature, the substance condenses into a liquid state.
Unique Properties at Critical Temperature
At the critical temperature, substances exhibit unique properties. For example, their density approaches zero, and the distinction between the liquid and gas phases becomes blurred. This phenomenon is known as the critical point.
Relationship Between Critical Temperature and Pressure
The critical temperature of a substance is directly related to its pressure. As pressure increases, the critical temperature also increases. This relationship is crucial in various industrial applications and scientific research.
Role of Critical Temperature in Superconductivity
Critical temperature plays a pivotal role in superconductivity, the phenomenon where certain materials can conduct electric current with zero resistance. Understanding the critical temperature is fundamental in the development of high-temperature superconductors.
Significance in Phase Transitions
Phase transitions, such as the melting or boiling of a substance, occur at specific temperatures. The critical temperature determines the conditions under which these transitions take place, making it a critical parameter in understanding material behavior.
Influence on Carbon Dioxide’s Environmental Impact
Carbon dioxide (CO2) undergoes a critical temperature around -78.5 degrees Celsius (-109.3 degrees Fahrenheit). This critical temperature is significant as it determines the conditions under which carbon dioxide can exist as a solid, liquid, or gas, impacting its environmental effects and storage methods.
Critical Temperature and High-Pressure Chemistry
In high-pressure chemistry, critical temperatures play a crucial role in studying the behavior of substances under extreme conditions. By understanding the critical temperature, scientists can explore new materials with remarkable properties.
Measurement and Calculation of Critical Temperature
Various experimental techniques and theoretical models are used to measure and calculate the critical temperature of different substances. These methods allow scientists to predict and manipulate material behavior, opening the door to numerous applications.
Understanding the 8 intriguing facts about critical temperature (Tc) provides a deeper insight into the behavior and properties of substances. Whether it’s predicting phase transitions or exploring new materials, critical temperature plays a fundamental role in the world of chemistry.
Conclusion
In conclusion, critical temperature (Tc) is a fascinating concept in the field of chemistry. It refers to the temperature at which a substance undergoes a phase transition, exhibiting unique and intriguing properties. Here are 8 intriguing facts about critical temperature:1. Definition: Critical temperature is the temperature at which a substance transitions from one phase to another, such as from a gas to a liquid or from a liquid to a solid.2. Influence on Properties: At temperatures below the critical temperature, substances generally exhibit distinct properties and behaviors. For example, water below its critical temperature behaves as a liquid, while above the critical temperature, it behaves as a gas.3. Importance in Supercritical Fluids: Supercritical fluids are substances that are above their critical temperatures and pressures, resulting in unique properties. These fluids have applications in various industries, including pharmaceuticals, food processing, and environmental remediation.4. Determining Factor for Phase Transitions: The critical temperature plays a significant role in determining the behavior of a substance during phase transitions. It determines whether a substance will condense, evaporate, or transition to a different phase.5. Role in Cryogenics: Critical temperature is crucial in the field of cryogenics, which involves the production and study of extremely low temperatures. Substances with low critical temperatures, like helium and nitrogen, are commonly used as cryogens.6. Relationship with Critical Pressure: Critical temperature is closely related to critical pressure. Together, they define the critical point of a substance, which represents the extreme conditions where both gas and liquid phases cease to exist.7. Research and Development: Critical temperature is an area of active research in materials science and chemistry. Scientists are continually studying ways to manipulate and control critical temperatures to develop new materials and enhance industrial processes.8. Practical Applications: Understanding critical temperature is essential for various practical applications, such as designing pressure vessels, optimizing chemical reactions, and developing advanced cooling systems.In conclusion, critical temperature is a fascinating and vital concept in the world of chemistry. It influences various aspects of substance behavior, phase transitions, and material development, making it a subject of ongoing research and exploration.
FAQs
Q: What is critical temperature in chemistry?
A: Critical temperature in chemistry refers to the temperature at which a substance undergoes a phase transition, resulting in unique properties and behaviors.
Q: How is critical temperature determined?
A: Critical temperature is determined through experimental observations and calculations. It is often measured at the point where the substance’s density changes abruptly during a phase transition.
Q: What happens at critical temperature?
A: At the critical temperature, substances experience significant changes in properties and behaviors. They can become supercritical fluids, losing their distinct gas or liquid phases.
Q: What are supercritical fluids?
A: Supercritical fluids are substances that exist above their critical temperatures and pressures. They have unique properties, such as high solvency and low viscosity, making them useful in various industrial applications.
Q: What is the significance of critical temperature in cryogenics?
A: Critical temperature is crucial in cryogenics as it determines the choice of cryogenic fluids. Substances with low critical temperatures, like helium and nitrogen, are commonly used as cryogens to achieve extremely low temperatures.
Q: How are critical temperature and critical pressure related?
A: Critical temperature and critical pressure are closely related. Together, they define the critical point of a substance, which represents the extreme conditions where both gas and liquid phases cease to exist.
Q: What are some practical applications of understanding critical temperature?
A: Understanding critical temperature is important in various practical applications. It helps in designing pressure vessels, optimizing chemical reactions, and developing advanced cooling systems, among others.
Q: Is critical temperature a subject of ongoing research?
A: Yes, critical temperature is an area of active research in materials science and chemistry. Scientists are continually exploring ways to manipulate and control critical temperatures to discover new materials and improve industrial processes.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
