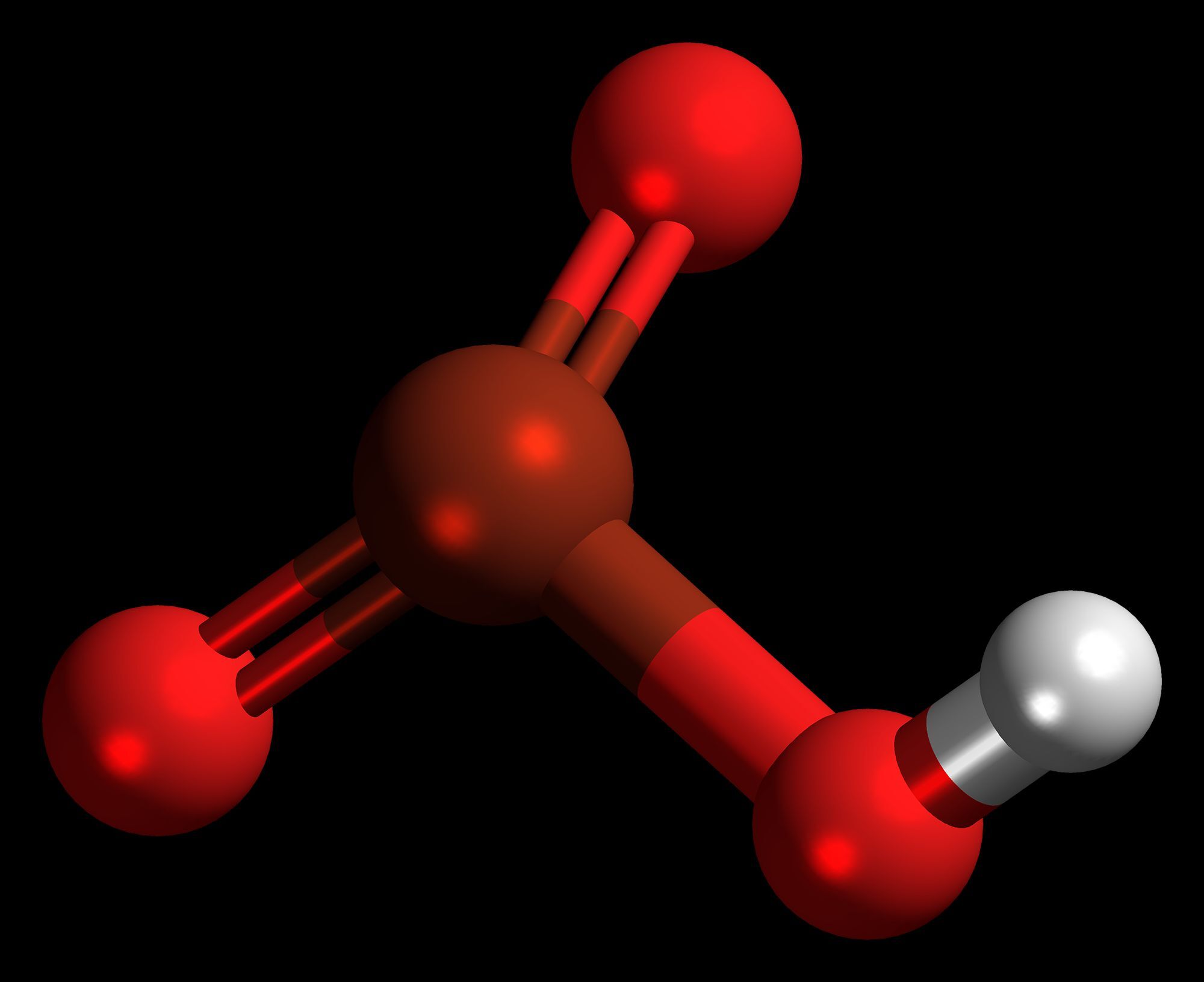
Bromic acid might sound like something out of a science fiction novel, but it's a real chemical compound with some intriguing properties. Bromic acid is a strong acid with the chemical formula HBrO3. It's not something you'd find in your kitchen, but it's important in chemistry labs and industrial processes. This acid is part of the halogen oxoacids family, which includes other acids like chloric and iodic acids. When dissolved in water, bromic acid forms bromate ions, which are used in various applications, including water treatment and chemical synthesis. However, handling bromic acid requires caution due to its corrosive nature. Understanding its properties and uses can help us appreciate its role in science and industry. Whether you're a budding chemist or just curious about the world of chemicals, bromic acid offers a glimpse into the fascinating interplay of elements and compounds.
Key Takeaways:
- Bromic acid is a powerful and reactive chemical compound used in various industrial applications, water treatment, and organic synthesis. Its unique properties make it a subject of ongoing scientific research and intrigue.
- Safety is crucial when handling bromic acid due to its corrosive nature and potential environmental impact. Understanding its properties and applications helps in responsible use and disposal.
What is Bromic Acid?
Bromic acid is a chemical compound with the formula HBrO₃. It's not something you encounter every day, but it plays a role in various chemical reactions. Let's dive into some fascinating facts about this compound.
-
Bromic acid is a strong acid. It can donate protons easily, making it quite reactive in chemical processes.
-
It contains bromine. Bromine is a halogen, similar to chlorine and iodine, and is known for its reactivity.
-
HBrO₃ is not stable in pure form. It tends to decompose into bromate and water, which is why it's often found in solution.
-
Used in chemical synthesis. Bromic acid can be used to produce bromates, which are important in various industrial applications.
-
It’s a powerful oxidizing agent. This means it can accept electrons from other substances, often used in oxidation reactions.
How is Bromic Acid Produced?
Producing bromic acid involves specific chemical reactions. Understanding its production helps in grasping its applications and handling.
-
Formed by dissolving bromine in water. This reaction produces a mixture of bromic acid and hydrobromic acid.
-
Can be synthesized from bromates. By reacting bromates with strong acids, bromic acid can be produced.
-
Electrochemical methods are used. Electrolysis of bromide solutions can lead to the formation of bromic acid.
-
Requires careful handling. Due to its reactivity, producing bromic acid must be done with caution to prevent unwanted reactions.
-
Not commonly produced in large quantities. Its instability makes it less practical for mass production compared to other acids.
Applications of Bromic Acid
While not as widely used as other acids, bromic acid has its niche applications, especially in industrial settings.
-
Used in water treatment. Bromates, derived from bromic acid, can help disinfect water.
-
Involved in analytical chemistry. It serves as a reagent for detecting certain substances due to its oxidizing properties.
-
Plays a role in organic synthesis. Bromic acid can help introduce bromine into organic compounds.
-
Used in explosives. Its oxidizing ability makes it useful in the production of certain explosive materials.
-
Limited use in laboratories. Due to its instability, it's not a common reagent but is used for specific reactions.
Safety and Handling of Bromic Acid
Handling bromic acid requires understanding its properties and potential hazards. Safety is paramount when dealing with such reactive substances.
-
Corrosive nature. Bromic acid can cause burns on contact with skin or eyes.
-
Produces toxic fumes. When decomposing, it can release harmful gases, necessitating proper ventilation.
-
Requires protective gear. Gloves, goggles, and lab coats are essential when working with bromic acid.
-
Should be stored properly. It needs to be kept in a cool, dry place away from incompatible substances.
-
Emergency procedures are crucial. Knowing how to handle spills or exposure is vital for safety.
Environmental Impact of Bromic Acid
Understanding the environmental implications of bromic acid helps in managing its use and disposal responsibly.
-
Can contribute to pollution. Improper disposal can lead to contamination of water sources.
-
Affects aquatic life. Bromates can be harmful to fish and other aquatic organisms.
-
Regulated by environmental agencies. There are guidelines for its use and disposal to minimize environmental impact.
-
Decomposes naturally. Over time, bromic acid breaks down, reducing its long-term environmental footprint.
-
Research on alternatives. Scientists are exploring less harmful substitutes for bromic acid in various applications.
Chemical Properties of Bromic Acid
The chemical properties of bromic acid define its behavior in reactions and its interactions with other substances.
-
Molecular weight is 128.91 g/mol. This is the combined weight of its atoms.
-
Colorless in solution. Bromic acid solutions are typically clear and colorless.
-
Soluble in water. It dissolves easily, forming an acidic solution.
-
Decomposes at high temperatures. Heat can cause it to break down into bromine and oxygen.
-
Has a pKa of -2. This indicates its strength as an acid, with a low pKa signifying a strong acid.
Historical Context of Bromic Acid
The history of bromic acid provides insight into its discovery and development over time.
-
First studied in the 19th century. Chemists began exploring its properties and potential uses.
-
Part of halogen acid research. It was studied alongside other halogen acids like hydrochloric and iodic acids.
-
Contributed to understanding oxidation. Research on bromic acid helped advance knowledge of oxidation reactions.
-
Used in early chemical experiments. Its reactivity made it a subject of interest for early chemists.
-
Evolved with industrial chemistry. As industries grew, so did the applications and understanding of bromic acid.
Bromic Acid in Modern Science
Today, bromic acid continues to be a subject of scientific research and exploration.
-
Studied for new applications. Researchers are looking into novel uses for bromic acid in various fields.
-
Part of environmental studies. Its impact on ecosystems is a focus of ongoing research.
-
Used in educational settings. Chemistry students learn about bromic acid as part of their studies on acids and bases.
-
Involved in green chemistry. Efforts are being made to use bromic acid in more sustainable ways.
-
Continues to intrigue scientists. Its unique properties make it a topic of interest in the scientific community.
Fun Facts about Bromic Acid
Let's wrap up with some lighter, fun facts about bromic acid that might surprise you.
-
Not found in nature. Bromic acid is a man-made compound, not occurring naturally.
-
Has a unique smell. It has a distinct, sharp odor that can be quite noticeable.
-
Used in fireworks. Its oxidizing properties can help create colorful displays.
-
Part of the periodic table family. As a bromine compound, it's related to other halogens like chlorine and iodine.
-
Featured in chemistry puzzles. Its complex reactions make it a favorite for chemistry enthusiasts.
-
Can change colors. When reacting, it can produce different colors depending on the substances involved.
-
Part of chemical folklore. Stories of its use in early experiments are shared among chemists.
-
Inspires curiosity. Its reactivity and properties spark interest in those studying chemistry.
-
Has a role in science fiction. Occasionally mentioned in sci-fi as a mysterious or powerful substance.
-
A symbol of chemistry's complexity. Bromic acid represents the intricate and fascinating world of chemical reactions.
Final Thoughts on Bromic Acid
Bromic acid, a fascinating compound, holds a significant place in chemistry. Known for its oxidizing properties, it plays a crucial role in various industrial applications. Despite its usefulness, handling this acid requires caution due to its reactive nature. It’s not something you’d find in everyday products, but its impact on chemical processes is undeniable. Understanding its properties and uses can provide insights into the broader world of inorganic chemistry. While it might not be a household name, bromic acid's contributions to scientific advancements are noteworthy. Whether you're a student, a professional, or just curious, knowing about such compounds enriches your knowledge of how the world works. Keep exploring and learning about these intriguing substances, as they often hold the key to innovative solutions in science and technology.
Frequently Asked Questions
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
